TeriflunomideCAS# 163451-81-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163451-81-8 | SDF | Download SDF |
| PubChem ID | 54684141 | Appearance | Powder |
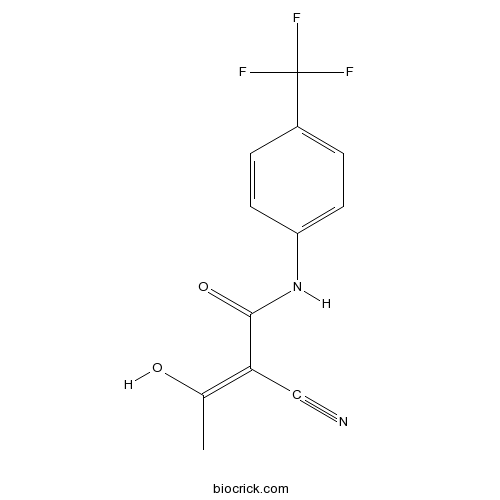
| Formula | C12H9F3N2O2 | M.Wt | 270.21 |
| Type of Compound | Impurities | Storage | Desiccate at -20°C |
| Synonyms | Flucyamide;A77 1726;108605-62-5;A 77-1726 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide | ||
| SMILES | CC(=C(C#N)C(=O)NC1=CC=C(C=C1)C(F)(F)F)O | ||
| Standard InChIKey | UTNUDOFZCWSZMS-YFHOEESVSA-N | ||
| Standard InChI | InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Teriflunomide Dilution Calculator

Teriflunomide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7008 mL | 18.5041 mL | 37.0083 mL | 74.0165 mL | 92.5206 mL |
| 5 mM | 0.7402 mL | 3.7008 mL | 7.4017 mL | 14.8033 mL | 18.5041 mL |
| 10 mM | 0.3701 mL | 1.8504 mL | 3.7008 mL | 7.4017 mL | 9.2521 mL |
| 50 mM | 0.074 mL | 0.3701 mL | 0.7402 mL | 1.4803 mL | 1.8504 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.3701 mL | 0.7402 mL | 0.9252 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Teriflunomide (also known as A77 1726), the active metabolite of an approved antirheumatic drug Leflunomide, is an emerging oral therapy for multiple sclerosis (MS). Teriflunomide has demonstrated anti-inflammatory, antiproliferative and immunosuppressive
Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.
In Vitro:Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzyme involved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high-avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus, teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes[1].
In Vivo:Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological, and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity[1].
References:
[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177-90.
- 2-[(1S,2S)-1-Ethyl-2-bezyloxypropyl]-2,4-dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-3H-1,2,4-triazol-3-one
Catalog No.:BCN9644
CAS No.:184177-83-1
- Toluene-4-sulfonic acid 5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-yl)methyltetrahydrofuran-3-ylmethyl ester
Catalog No.:BCN9643
CAS No.:149809-43-8
- 3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester
Catalog No.:BCN9642
CAS No.:211915-84-3
- 6-Des(1-methyl-2-benzimidazolyl)-6-carboxy telmisartan
Catalog No.:BCN9641
CAS No.:884330-12-5
- 2-Iodo-3-oxo-4-azaandrostane-17-carboxylic acid
Catalog No.:BCN9640
CAS No.:104239-97-6
- Diethyl 2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate 4-methylbenzenesulfonate
Catalog No.:BCN9639
CAS No.:165049-28-5
- Norgestrel
Catalog No.:BCN9638
CAS No.:6533-00-2
- Methyl 1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetra-hydro-1H-pyrido[3,4-b]indole-3-carboxylate
Catalog No.:BCN9637
CAS No.:171489-59-1
- cis-1,2,3,4-Tetrahydro-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride
Catalog No.:BCN9636
CAS No.:171752-68-4
- Telmisartan impurity G
Catalog No.:BCN9635
CAS No.:144702-27-2
- Telmisartan amide
Catalog No.:BCN9634
CAS No.:915124-86-6
- 2-(2-Aminopropan-2-yl)-N-(4-fluorobenzyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidine-4-carboxamide
Catalog No.:BCN9633
CAS No.:518048-03-8
- 5-[2-[[(4-Methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-3-(phenylmethoxy)-2-[(phenylmethoxy)methyl]cyclopentanol
Catalog No.:BCN9646
CAS No.:142217-78-5
- tert-Butyl 6-[(1E)-2-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]ethenyl]-2,2-dimethyl-1,3-dioxane-4-acetate
Catalog No.:BCN9647
CAS No.:289042-12-2
- 1,2,3,4-Tetrahydro-1-acetylquinoline
Catalog No.:BCN9648
CAS No.:4169-19-1
- Benzalacetone
Catalog No.:BCN9649
CAS No.:122-57-6
- Bisdemethoxycucurmin
Catalog No.:BCN9650
CAS No.:24939-16-0
- N,N'-Bis(2-hydroxyethyl)ethylenediamine
Catalog No.:BCN9651
CAS No.:4439-20-7
- 1-Benzyl D-aspartate
Catalog No.:BCN9652
CAS No.:79337-40-9
- 17-Ethylendioxyandrosta-1,4-dien-3-one
Catalog No.:BCN9653
CAS No.:2398-63-2
- Acid Orange 7
Catalog No.:BCN9654
CAS No.:633-96-5
- Saccharin sodium
Catalog No.:BCN9655
CAS No.:128-44-9
- Orange IV
Catalog No.:BCN9656
CAS No.:554-73-4
- Ethyl 3-(4-(methylamino)-3-nitro-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCN9657
CAS No.:429659-01-8
Twin pregnancy outcome following teriflunomide treatment in a relapsing-remitting multiple sclerosis patient: A case report.[Pubmed:32664171]
Medicine (Baltimore). 2020 Jul 10;99(28):e21212.
RATIONALE: Teriflunomide is a disease-modifying drug that has been approved for treatment of relapsing-remitting multiple sclerosis. Due to its teratogenic effect in animals, however, it is not recommended during pregnancy. For this reason, effective contraception must be used during its administration. When an unscheduled pregnancy occurs during therapy, patients must undergo a cholestyramine procedure for rapid flushing of the drug. PATIENT CONCERNS: We describe the case of a 35-year-old female patient suffering diagnosed with relapsing-remitting multiple sclerosis at the age of 20. The patient as a result of side effects of previous therapies started taking Teriflunomide. DIAGNOSIS: Despite recommendations for the use of contraceptives, the patient became pregnant during drug therapy. Pregnancy occurred 12 months after initiating Teriflunomide treatment. INTERVENTIONS: Therapy with Teriflunomide was immediately suspended and cholestyramine was prescribed (8 g 3 times a day, for 11 days) to flush out any residual drug from the body. OUTCOMES: Despite an 8-week exposure to teriflumomide during gestation, the patient gave birth to healthy twin girls at 35 week. Controls carried out after birth did not reveal any malformation or genetic and chromosomal abnormality. At a 5-month pediatric specialist check both babies were healthy and growing regularly. CONCLUSION: This shows that even if there is evidence of teratogenic effects in animals, an 8-week exposure to teraflunomide >0.02 mg/L did not have effects on the newborn.
Management of Idiopathic CNS inflammatory diseases during the COVID-19 pandemic: Perspectives and strategies for continuity of care from a South East Asian Center with limited resources.[Pubmed:32653804]
Mult Scler Relat Disord. 2020 Jul 3;44:102353.
The Covid-19 pandemic poses a grave health management challenge globally of unprecedented nature. Management of idiopathic Central Nervous system inflammatory disorders (iCNSID) such as Multiple sclerosis, Neuromyelitis optica and its spectrum disorders and related conditions during this pandemic needs to be addressed with affirmative and sustainable strategies in order to prevent disease related risks, medication related complications and possible COVID-19 disease associated effects. Global international iCNSIDs agencies and recent publications are attempting to address this but such guidance is not available in South East Asia. Here we outline prospectively qualitatively and quantitatively novel strategies at a tertiary center in Malaysia catering for neuroimmunological disorders despite modest resources during this pandemic. In this retrospective study with longitudinal follow-up, we describe stratification of patients for face to face versus virtual visits in the absence of formal teleneurology, stratification of patients for treatment according to disease activity, rescheduling, deferring initiation or extending treatment intervals of certain disease modifying therapies(DMT's) or immunosuppressants(IS), especially those producing lymphocyte depletion in MS and the continuation of IS in patients with NMO/NMOSD. Furthermore, we highlight the use off-label treatments such as Intravenous immunoglobulins/rituximab,bridging interferons/Teriflunomide temporarily replacing more potent DMT choices,supply challenges of IS/DMT's and tailoring blood watches and neuroimaging surveillance based on the current health needs to stave off the pandemic and prevent at risk patients with iCNSID/health care workers from possibly being exposed to the COVID-19.
MSCOVID19: Using social media to achieve rapid dissemination of health information.[Pubmed:32629402]
Mult Scler Relat Disord. 2020 Jun 24;45:102338.
BACKGROUND AND OBJECTIVE: The global COVID-19 pandemic creates an obvious acute health care resourcing and response problem. The different timing of pandemic peak in geographically distinct locations creates a short window of response opportunity. Rapid dissemination of medical information from early affected areas to later ones is therefore crucial to optimise planning. Formulating the best system response for at-risk patient populations is especially complex. People with multiple sclerosis (pwMS) are exposed to long-term immunosuppressive disease modifying treatments (DMTs) and, in theory, could be at increased risk of contracting the virus and developing complications. Social media, such as Twitter, can provide a global platform to rapidly share information and individual experiences. METHODS AND RESULTS: This report summarizes the case experience of pwMS with COVID-19 infection in the first month of the pandemic as reported on Twitter using the #MSCOVID19 hashtag. 26 individual cases of COVID-19 in pwMS were reported from Europe and the United States of America. The cases involved a combination of relapsing and progressive MS phenotypes treated with a range of DMT (5 anti CD20 therapy, 4 cladribine, 4 fingolimod, 4 injectables, 3 alemtuzumab, 2 dimethyl fumarate, 2 untreated, 1 Teriflunomide, 1 natalizumab). The cases shared present the earliest reported data on outcomes of COVID-19 infection in pwMS. Whilst limited, the cautiously reassuring nature of these early cases assisted in clinical management by allowing neurologists to continuously reassess their approach to DMT management.
Management of central nervous system demyelinating diseases during the coronavirus disease 2019 pandemic: a practical approach.[Pubmed:32609290]
Arq Neuropsiquiatr. 2020 Jul 1. pii: S0004-282X2020005015202.
BACKGROUND: The novel coronavirus disease 2019 (COVID-19) pandemic poses a potential threat to patients with autoimmune disorders, including multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). Such patients are usually treated with immunomodulatory or immunosuppressive agents, which may tamper with the organism's normal response to infections. Currently, no consensus has been reached on how to manage MS and NMOSD patients during the pandemic. OBJECTIVE: To discuss strategies to manage those patients. METHODS: We focus on how to 1) reduce COVID-19 infection risk, such as social distancing, telemedicine, and wider interval between laboratory testing/imaging; 2) manage relapses, such as avoiding treatment of mild relapse and using oral steroids; 3) manage disease-modifying therapies, such as preference for drugs associated with lower infection risk (interferons, glatiramer, Teriflunomide, and natalizumab) and extended-interval dosing of natalizumab, when safe; 4) individualize the chosen MS induction-therapy (anti-CD20 monoclonal antibodies, alemtuzumab, and cladribine); 5) manage NMOSD preventive therapies, including initial therapy selection and current treatment maintenance; 6) manage MS/NMOSD patients infected with COVID-19. CONCLUSIONS: In the future, real-world case series of MS/NMOSD patients infected with COVID-19 will help us define the best management strategies. For the time being, we rely on expert experience and guidance.
Monitoring of Human Herpesvirus 6 infection in the management of drug reaction with eosinophilia and systemic symptoms.[Pubmed:32608082]
Clin Exp Dermatol. 2020 Jul 1.
We read with great interest the article recently published by Choudhary et al,(1) reporting a fatal case of acute hepatitis failure as complication of a Teriflunomide-induced drug reaction with eosinophilia and systemic symptoms (DRESS). This hepatitis failure developed after the patient suddenly stopped corticosteroids.
Effects of Teriflunomide on B Cell Subsets in MuSK-Induced Experimental Autoimmune Myasthenia Gravis and Multiple Sclerosis.[Pubmed:32597289]
Immunol Invest. 2020 Jun 29:1-14.
Antigen-specific immune responses are crucially involved in both multiple sclerosis (MS) and myasthenia gravis (MG). Teriflunomide is an immunomodulatory agent approved for treatment of MS through inhibition of lymphocyte proliferation. MG associated with muscle-specific tyrosine kinase (MuSK) antibodies often manifests with a severe disease course, prompting development of effective treatment methods. To evaluate whether Teriflunomide treatment may ameliorate MuSK-autoimmunity, experimental autoimmune MG (EAMG) was induced by immunizing C57BL/6 (B6) mice three times with MuSK in complete Freund's adjuvant (CFA) (n = 17). MuSK-immunized mice were treated daily with Teriflunomide (n = 8) or PBS (n = 9) starting from the third immunization (week 8) to termination (week 14). Clinical severity of EAMG was monitored. Immunological alterations were evaluated by measurement of anti-MuSK IgG, neuromuscular junction deposits, and flow cytometric analysis of lymph node cells. In MS patients under Teriflunomide treatment, the peripheral blood B cell subset profile was analyzed. B6 mice treated with Teriflunomide displayed relatively preserved body weight, lower EAMG prevalence, reduced average clinical grades, higher inverted screen scores, diminished anti-MuSK antibody and NMJ deposit levels. Amelioration of EAMG findings was associated with reduced memory B cell ratios in the lymph nodes. Similarly, MS patients under Teriflunomide treatment showed reduced memory B cell, plasma cell, and plasmablast ratios. Teriflunomide treatment has effectively ameliorated MuSK-autoimmunity and thus may putatively be used in long-term management of MuSK-MG as an auxiliary treatment method. Teriflunomide appears to exert beneficial effects through inhibition of effector B cells.
Medication adherence/persistence among patients with active multiple sclerosis in Finland.[Pubmed:32559310]
Acta Neurol Scand. 2020 Jun 19.
OBJECTIVES: To explore adherence, persistence, and treatment patterns in patients with multiple sclerosis (MS) in Finland treated with disease-modifying therapies (DMTs) for active MS in 2005-2018. MATERIALS AND METHODS: The study cohort was identified using the Drug Prescription Register of Social Insurance Institute, Finland. All patients had at least one prescription of glatiramer acetate (GA), beta-interferons, Teriflunomide, or delayed-release dimethyl fumarate (DMF). Adherence was calculated using proportion of days covered (PDC) (cutoff>/=0.8). Time to non-persistence was calculated by the number of days on index DMT treatment before the first treatment gap (>/=90 days) or switch, and analyzed with time-to-event methodology. RESULTS: The cohort included 7474 MS patients (72.2% female; mean age 38.9 years). Treatment switches were steady over 2005-2012, peaking in 2015. PDC means (standard deviations) were GA, 0.87 (0.17); beta-interferons, 0.88 (0.15); DMF, 0.89 (0.14); Teriflunomide, 0.93 (0.10). Adherence frequencies were GA, 78.4%; beta-interferons, 81.3%; DMF, 86.9%; Teriflunomide, 91.7%. Logistic regression showed that age group, DMT and the starting year, sex, and hospital district independently affected adherence. Patients receiving Teriflunomide and DMF, males, and older patients were more likely to persist on treatment. There was no difference in persistence between patients prescribed Teriflunomide and DMF, or between GA and beta-interferons. CONCLUSIONS: Oral DMTs had greater adherence and persistence than injectable DMTs.
Teriflunomide induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome.[Pubmed:32557734]
Clin Exp Dermatol. 2020 Jun 17.
A 27-year old female presented with a 3 day history of fever, itchy maculopapular rash progressing cephalocaudally and facial swelling. She was a known case of multiple sclerosis (MS) taking oral Teriflunomide 14 mg for past 27 days. She was not taking any other drug. Symptom appeared 3 weeks after initiation of drug. Clinical examination revealed dusky maculopapular rash involving trunk (Figure 1a) and extremities (Figure 1b) and diffuse facial swelling with erythema and scaling.
Molecular Effects of FDA-Approved Multiple Sclerosis Drugs on Glial Cells and Neurons of the Central Nervous System.[Pubmed:32545828]
Int J Mol Sci. 2020 Jun 13;21(12). pii: ijms21124229.
Multiple sclerosis (MS) is characterized by peripheral and central inflammatory features, as well as demyelination and neurodegeneration. The available Food and Drug Administration (FDA)-approved drugs for MS have been designed to suppress the peripheral immune system. In addition, however, the effects of these drugs may be partially attributed to their influence on glial cells and neurons of the central nervous system (CNS). We here describe the molecular effects of the traditional and more recent FDA-approved MS drugs Fingolimod, Dimethyl Fumarate, Glatiramer Acetate, Interferon-beta, Teriflunomide, Laquinimod, Natalizumab, Alemtuzumab and Ocrelizumab on microglia, astrocytes, neurons and oligodendrocytes. Furthermore, we point to a possible common molecular effect of these drugs, namely a key role for NFkappaB signaling, causing a switch from pro-inflammatory microglia and astrocytes to anti-inflammatory phenotypes of these CNS cell types that recently emerged as central players in MS pathogenesis. This notion argues for the need to further explore the molecular mechanisms underlying MS drug action.
Dimethyl fumarate vs Teriflunomide: an Italian time-to-event data analysis.[Pubmed:32506391]
J Neurol. 2020 Jun 6. pii: 10.1007/s00415-020-09959-1.
BACKGROUND: The introduction of oral disease-modifying therapies (DMTs) for relapsing-remitting multiple sclerosis (RRMS) changed algorithms of RRMS treatment. OBJECTIVES: To compare the effectiveness of treatment with dimethyl fumarate (DMF) and Teriflunomide (TRF) in a large multicentre Italian cohort of RRMS patients. MATERIALS AND METHODS: Patients with RRMS who received treatment with DMF and TRF between January 1st, 2012 and December 31st, 2018 from twelve MS centers were identified. The events investigated were "time-to-first-relapse", "time-to-Magnetic-Resonance-Imaging (MRI)-activity" and "time-to-disability-progression". RESULTS: 1445 patients were enrolled (1039 on DMF, 406 on TRF) and followed for a median of 34 months. Patients on TRF were older (43.5 +/- 8.6 vs 38.8 +/- 9.2 years), with a predominance of men and higher level of disability (p < 0.001 for all). Patients on DMF had a higher number of relapses and radiological activity (p < .05) at baseline. Time-varying Cox-model for the event "time-to-first relapse" revealed that no differences were found between the two groups in the first 38 months of treatment (HRt < 38DMF = 0.73, CI = 0.52 to 1.03, p = 0.079). When the time-on-therapy exceeds 38 months patients on DMF had an approximately 0.3 times lower relapse hazard risk than those who took TRF (HRt>38DMF = 3.83, CI = 1.11 to 13.23, p = 0.033). Both DMTs controlled similarly MRI activity and disability progression. CONCLUSIONS: Patients on DMF had higher relapse-free survival time than TRF group after the first 38 months on therapy.
COVID-19 in teriflunomide-treated patients with multiple sclerosis.[Pubmed:32494856]
J Neurol. 2020 Jun 3. pii: 10.1007/s00415-020-09944-8.
The outbreak of a severe acute respiratory syndrome caused by a novel coronavirus (COVID-19), has raised health concerns for patients with multiple sclerosis (MS) who are commonly on long-term immunotherapies. Managing MS during the pandemic remains challenging with little published experience and no evidence-based guidelines. We present five Teriflunomide-treated patients with MS who subsequently developed active COVID-19 infection. The patients continued Teriflunomide therapy and had self-limiting infection, without relapse of their MS. These observations have implications for the management of MS in the setting of the COVID-19 pandemic.


