PowellineCAS# 7363-25-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

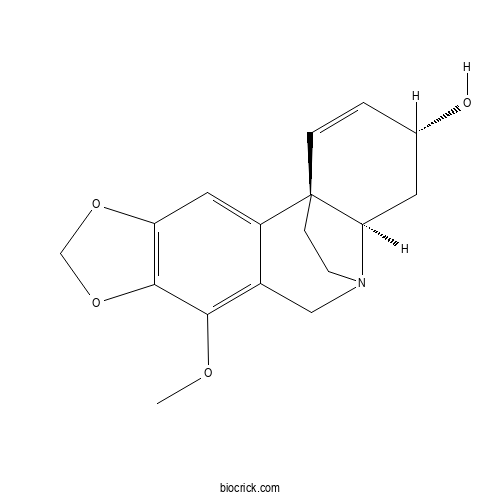
| Cas No. | 7363-25-9 | SDF | Download SDF |
| PubChem ID | 443669 | Appearance | Powder |
| Formula | C17H19NO4 | M.Wt | 301.34 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,13R,15R)-9-methoxy-5,7-dioxa-12-azapentacyclo[10.5.2.01,13.02,10.04,8]nonadeca-2,4(8),9,16-tetraen-15-ol | ||
| SMILES | COC1=C2CN3CCC4(C3CC(C=C4)O)C2=CC5=C1OCO5 | ||
| Standard InChIKey | VXTCKUJRGBGTEH-NCAQKEMTSA-N | ||
| Standard InChI | InChI=1S/C17H19NO4/c1-20-15-11-8-18-5-4-17(3-2-10(19)6-14(17)18)12(11)7-13-16(15)22-9-21-13/h2-3,7,10,14,19H,4-6,8-9H2,1H3/t10-,14+,17+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Powelline Dilution Calculator

Powelline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3185 mL | 16.5926 mL | 33.1851 mL | 66.3702 mL | 82.9628 mL |
| 5 mM | 0.6637 mL | 3.3185 mL | 6.637 mL | 13.274 mL | 16.5926 mL |
| 10 mM | 0.3319 mL | 1.6593 mL | 3.3185 mL | 6.637 mL | 8.2963 mL |
| 50 mM | 0.0664 mL | 0.3319 mL | 0.6637 mL | 1.3274 mL | 1.6593 mL |
| 100 mM | 0.0332 mL | 0.1659 mL | 0.3319 mL | 0.6637 mL | 0.8296 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2',4',6'-Trihydroxydihydrochalcone
Catalog No.:BCX0182
CAS No.:1088-08-0
- Lycoctonine
Catalog No.:BCX0181
CAS No.:26000-17-9
- N-Tetracosanoyltyramine
Catalog No.:BCX0180
CAS No.:113122-70-6
- Vesuvianic acid
Catalog No.:BCX0179
CAS No.:63090-99-3
- β-Cembrenediol
Catalog No.:BCX0178
CAS No.:57605-81-9
- α-Cembrenediol
Catalog No.:BCX0177
CAS No.:57605-80-8
- Anthriscifoldine B
Catalog No.:BCX0176
CAS No.:1158213-52-5
- 3-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCX0175
CAS No.:27391-18-0
- Macarangin
Catalog No.:BCX0174
CAS No.:129385-65-5
- 5,5'-Oxybis(pyrrolidin-2-one)
Catalog No.:BCX0173
CAS No.:73123-79-2
- (-)-Byakangelicin
Catalog No.:BCX0172
CAS No.:23517-02-4
- (+)-3-Hydroxy-p-menth-1-en-6-one
Catalog No.:BCX0171
CAS No.:120850-08-0
- Amentoflavone 7'',4'''-dimethyl ether
Catalog No.:BCX0184
CAS No.:34293-14-6
- 1-O-p-Coumaroyl-3-O-feruloylglycerol
Catalog No.:BCX0185
CAS No.:108026-21-7
- Gnemontanin F
Catalog No.:BCX0186
CAS No.:2210293-19-7
- (S)-3-Hydroxy-1,5-diphenylpentan-1-one
Catalog No.:BCX0187
CAS No.:163086-00-8
- 1α,7α,10αH-Guaia-4,11-dien-3-one
Catalog No.:BCX0188
CAS No.:120838-14-4
- Bisacurone B
Catalog No.:BCX0189
CAS No.:127214-85-1
- Denticulatain B
Catalog No.:BCX0190
CAS No.:1842404-33-4
- Petiolin G
Catalog No.:BCX0191
CAS No.:1204251-11-5
- 2β,15α-Dihydroxy-ent-kaur-16-ene
Catalog No.:BCX0192
CAS No.:34302-36-8
- Neoolivil
Catalog No.:BCX0193
CAS No.:1277182-38-3
- Hippadine
Catalog No.:BCX0194
CAS No.:52886-06-3
- Gelse-norursane B
Catalog No.:BCX0195
CAS No.:2072868-77-8
Chiral Bisphosphine-Catalyzed Asymmetric Staudinger/aza-Wittig Reaction: An Enantioselective Desymmetrizing Approach to Crinine-Type Amaryllidaceae Alkaloids.[Pubmed:38642063]
J Am Chem Soc. 2024 Apr 20.
An unprecedented chiral bisphosphine-catalyzed asymmetric Staudinger/aza-Wittig reaction of 2,2-disubstituted cyclohexane-1,3-diones is reported, enabling the facile access of a broad range of cis-3a-arylhydroindoles in high yields with excellent enantioselectivities. The key to the success of this work relies on the first application of chiral bisphosphine DuanPhos to the asymmetric Staudinger/aza-Wittig reaction. An effective reductive system has been established to address the challenging P(V) horizontal lineO/P(III) redox cycle associated with the chiral bisphosphine catalyst. In addition, comprehensive experimental and computational investigations were carried out to elucidate the mechanism of the asymmetric reaction. Leveraging the newly developed chemistry, the enantioselective total syntheses of several crinine-type Amaryllidaceae alkaloids, including (+)-Powelline, (+)-buphanamine, (+)-vittatine, and (+)-crinane, have been accomplished with remarkable conciseness and efficiency.
Cytotoxic Activity of Amaryllidaceae Plants against Cancer Cells: Biotechnological, In Vitro, and In Silico Approaches.[Pubmed:36985571]
Molecules. 2023 Mar 13;28(6):2601.
Cancer is a major cause of death and an impediment to increasing life expectancy worldwide. With the aim of finding new molecules for chemotherapeutic treatment of epidemiological relevance, ten alkaloid fractions from Amaryllidaceae species were tested against six cancer cell lines (AGS, BT-549, HEC-1B, MCF-7, MDA-MB 231, and PC3) with HaCat as a control cell line. Some species determined as critically endangered with minimal availability were propagated using in vitro plant tissue culture techniques. Molecular docking studies were carried out to illustrate binding orientations of the 30 Amaryllidaceae alkaloids identified in the active site of some molecular targets involved with anti-cancer activity for potential anti-cancer drugs. In gastric cancer cell line AGS, the best results (lower cell viability percentages) were obtained for Crinum jagus (48.06 +/- 3.35%) and Eucharis bonplandii (45.79 +/- 3.05%) at 30 microg/mL. The research focused on evaluating the identified alkaloids on the Bcl-2 protein family (Mcl-1 and Bcl-xL) and HK2, where the in vitro, in silico and statistical results suggest that Powelline and buphanidrine alkaloids could present cytotoxic activity. Finally, combining experimental and theoretical assays allowed us to identify and characterize potentially useful alkaloids for cancer treatment.
Antibacterial and Anticancer Activity and Untargeted Secondary Metabolite Profiling of Crude Bacterial Endophyte Extracts from Crinum macowanii Baker Leaves.[Pubmed:33488726]
Int J Microbiol. 2020 Dec 10;2020:8839490.
This study isolated and identified endophytic bacteria from the leaves of Crinum macowanii and investigated the potential of the bacterial endophyte extracts as antibacterial and anticancer agents and their subsequent secondary metabolites. Ethyl acetate extracts from the endophytes and the leaves (methanol: dichloromethane (1 : 1)) were used for antibacterial activity against selected pathogenic bacterial strains by using the broth microdilution method. The anticancer activity against the U87MG glioblastoma and A549 lung carcinoma cells was determined by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Bacterial endophytes that were successfully isolated from C. macowanii leaves include Raoultella ornithinolytica, Acinetobacter guillouiae, Pseudomonas sp., Pseudomonas palleroniana, Pseudomonas putida, Bacillus safensis, Enterobacter asburiae, Pseudomonas cichorii, and Arthrobacter pascens. Pseudomonas cichorii exhibited broad antibacterial activity against both Gram-negative and Gram-positive pathogenic bacteria while Arthrobacter pascens displayed the least MIC of 0.0625 mg/mL. Bacillus safensis crude extracts were the only sample that showed notable cell reduction of 50% against A549 lung carcinoma cells at a concentration of 100 mug/mL. Metabolite profiling of Bacillus safensis, Pseudomonas cichorii, and Arthrobacter pascens crude extracts revealed the presence of known antibacterial and/or anticancer agents such as lycorine (1), angustine (2), crinamidine (3), vasicinol (4), and Powelline. It can be concluded that the crude bacterial endophyte extracts obtained from C. macowanii leaves can biosynthesize bioactive compounds and can be bioprospected for medical application into antibacterial and anticancer agents.
Alkaloids of Amaryllidaceae as Inhibitors of Cholinesterases (AChEs and BChEs): An Integrated Bioguided Study.[Pubmed:29044771]
Phytochem Anal. 2018 Mar;29(2):217-227.
INTRODUCTION: Enzymatic inhibition of acetylcholinesterase (AChE) is an essential therapeutic target for the treatment of Alzheimer's disease (AD) and AChE inhibitors are the first-line drugs for it treatment. However, butyrylcholinesterase (BChE), contributes critically to cholinergic dysfunction associated with AD. Thus, the development of novel therapeutics may involve the inhibition of both cholinesterase enzymes. OBJECTIVE: To evaluate, in an integrated bioguided study, cholinesterases alkaloidal inhibitors of Amaryllidaceae species. METHODOLOGY: The proposed method combines high-performance thin-layer chromatography (HPTLC) with data analysis by densitometry, enzymatic bioautography with different AChEs and BChEs, the detection of bioactive molecules through gas chromatography mass spectrometry (GC-MS) analysis of spots of interest, and theoretical in silico studies. RESULTS: To evaluate the bioguided method, the AChE and BChE inhibitory activities of seven Amaryllidaceae plant extracts were evaluated. The alkaloid extracts of Eucharis bonplandii exhibited a high level of inhibitory activity (IC(50) = 0.72 +/- 0.05 mug/mL) against human recombinant AChE (hAChE). Regarding human serum BChE (hBChE), the bulb and leaf extracts of Crinum jagus had the highest activity (IC(50) = 8.51 +/- 0.56 mug/mL and 11.04 +/- 1.21 mug/mL, respectively). In the HPTLC spots with high inhibitory activity, several alkaloids were detected using GC-MS, and some of these alkaloids were identified. Galanthamine, galanthamine N-oxide and Powelline should be the most prominent inhibitors of substrate accommodation in the active site of the Torpedo californica AChE (TcAChE), hAChE and hBChE enzymes. CONCLUSIONS: These results are evidence of the chemical relevance of the Colombian's Amaryllidaceae species for the inhibition of cholinesterases and as potent sources for the palliative treatment of AD. Copyright (c) 2017 John Wiley & Sons, Ltd.
In-vitro evaluation of the P-glycoprotein interactions of a series of potentially CNS-active Amaryllidaceae alkaloids.[Pubmed:23058055]
J Pharm Pharmacol. 2012 Nov;64(11):1667-77.
OBJECTIVES: Drug compounds interacting with the blood-brain barrier efflux transporter P-glycoprotein (P-gp) might have limited access to brain tissue. The aim of the present study was to evaluate whether nine potentially CNS-active Amaryllidaceae alkaloids of the crinine, lycorine and galanthamine types interact with P-gp. METHODS: Alkaloids with inhibitory activity towards either the serotonin reuptake transporter or acetylcholinesterase, or both, were investigated using the calcein-AM efflux assay in Madin-Darby canine kidney cells transfected with human multidrug resistance transporter 1. KEY FINDINGS: Powelline and 6-hydroxycrinamine showed an interaction with P-gp, with IC50 values between 300 and 500 microM. 3-O-Acetylhamayne showed a weaker interaction, with an IC50 value above 3 mM. Epibuphanisine, lycorine, 1-epi-deacetylbowdenisine, papyramine and galanthamine all showed weak or no interaction with P-gp. There was no observed correlation between alkaloid type and P-gp interaction. CONCLUSIONS: Structurally similar compounds such as crinine and epibuphanisine showed very different P-gp interactions, highlighting the difficulty in predicting P-gp interactions. Epibuphanisine has previously shown activity in the serotonin reuptake transporter assay and may therefore serve as a lead for serotonin reuptake transporter active compounds. The most potent compound in the acetylcholinesterase assay, the marketed drug compound galanthamine (Reminyl), showed no interaction with P-gp.
Total synthesis of (+/-)-powelline and (+/-)-buphanidrine.[Pubmed:20175516]
Org Lett. 2010 Mar 19;12(6):1252-4.
The total synthesis of (+/-)-Powelline (13 linear steps in an overall yield of 6%) and (+/-)-buphanidrine (14 linear steps and a 6% overall yield) and has been achieved using a novel approach to the crinane skeleton. An organocatalytic oxidative coupling allowed direct construction of the key quaternary carbon-to-aryl bond in high yield allowing rapid access to the target alkaloids.
Inhibition of [3H]citalopram binding to the rat brain serotonin transporter by Amaryllidaceae alkaloids.[Pubmed:16557463]
Planta Med. 2006 Apr;72(5):470-3.
Twenty-one Amaryllidaceae alkaloids isolated from different Amaryllidaceae species were investigated for their affinity to the serotonin reuptake transport protein and for GABA(A)-benzodiazepine receptor binding. Cherylline (21), crinamine (7), crinine (1), epibuphanisine (2), epivittatine (6), maritidine (11), O-methylmaritidine (12), Powelline (3), 1-O-acetyllycorine (18) and tazettine ( 13) showed affinity to the serotonin reuptake transport protein. Cherylline (21) and epivittatine (6) yielded the highest activity among the group. No GABA(A)-benzodiazepine receptor binding activity was exhibited by the alkaloids tested.
Alkaloid production in Crinum moorei cultures.[Pubmed:14640535]
J Nat Prod. 2003 Nov;66(11):1524-6.
The alkaloids cherylline (1), crinamidine (2), crinine (3), epibuphanisine (4), lycorine (5), Powelline (6), undulatine (7), 1-epideacetylbowdensine (8), and 3-O-acetylhamayne (9) were identified in the in vitro propagated bulblets of Crinum moorei. In addition, crinine, Powelline, and undulatine were detected in the solidified Murashige and Skoog (MS) medium. The identity of the alkaloids was confirmed by comparing retention times and mass spectra with known samples. Light, as well as benzyladenine (BA) and charcoal supplementation of the tissue culture medium, influenced the levels of specific alkaloids in both the bulblets and media.
Organ-to-organ and seasonal variation in alkaloids from Crinum macowanii.[Pubmed:12385872]
Fitoterapia. 2002 Oct;73(6):490-5.
The distribution and seasonal variation of alkaloids from Crinum macowanii were investigated. The alkaloids lycorine, 1-O-acetyllycorine, crinine, Powelline, crinamine, crinamidine, 3-O-acetylhamayne, 1-epideacetylbowdensine and cherylline were isolated from this plant using gas chromatography. Significant organ-to-organ variations were detected for the alkaloids crinine, lycorine, Powelline, crinamidine, 3-O-acetylhamayne and crinamine. Crinine, crinamidine and 1-epideacetylbowdensine showed significant seasonal variation.
Separation of alkaloids by pH-zone-refining counter-current chromatography.[Pubmed:7842146]
J Chromatogr A. 1994 Nov 18;685(2):259-62.
pH-Zone-refining counter-current chromatography was applied to the separation of alkaloids from a crude extract of Crinum moorei using a multilayer coil planet centrifuge. After methyl tert.-butyl ether and water were equilibrated, triethylamine (5-10 mM) was added to the organic phase and hydrochloric acid (5-10 mM) to the aqueous phase. The separation was performed by eluting the column with either the organic phase (displacement mode) or the aqueous phase (reverse-displacement mode) while the other phase was used as the stationary phase. From 3 g of the extract, crinine, Powelline and crinamidine were separated in 2.5-7 h with minimum overlapping.
Cytotoxic activity of Amaryllidaceae alkaloids from Crinum augustum and Crinum bulbispermum.[Pubmed:1798796]
Planta Med. 1991 Oct;57(5):437-9.
The cytotoxic activity of five minor Amaryllidaceae alkaloids and one flavan isolated from Crinum augustum Rox and Crinum bulbispermum Milne were tested on human leukemic Molt 4 cells. Whereas the crinine-type alkaloids (6 alpha-hydroxycrinine, Powelline) and the new type augustamine did not even inhibit the growth of Molt 4 cells, the lycorine-type alkaloid (pratorinine) and the crinine-type alkaloid (6 alpha-hydroxybuphanisine) showed a moderate cytotoxic activity and the flavan (4'-hydroxy-7-methoxyflavan) showed an important cytotoxic effect.


