PrimeveroseCAS# 26531-85-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26531-85-1 | SDF | Download SDF |
| PubChem ID | 5460006 | Appearance | Powder |
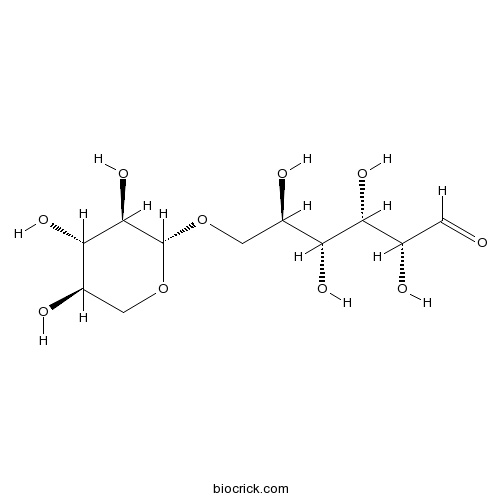
| Formula | C11H20O10 | M.Wt | 312.27 |
| Type of Compound | Other NPs | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4R,5R)-2,3,4,5-tetrahydroxy-6-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyhexanal | ||
| SMILES | C1C(C(C(C(O1)OCC(C(C(C(C=O)O)O)O)O)O)O)O | ||
| Standard InChIKey | XOPPYWGGTZVUFP-DLWPFLMGSA-N | ||
| Standard InChI | InChI=1S/C11H20O10/c12-1-4(13)7(16)8(17)5(14)2-20-11-10(19)9(18)6(15)3-21-11/h1,4-11,13-19H,2-3H2/t4-,5+,6+,7+,8+,9-,10+,11+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Primeverose Dilution Calculator

Primeverose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2024 mL | 16.0118 mL | 32.0236 mL | 64.0471 mL | 80.0589 mL |
| 5 mM | 0.6405 mL | 3.2024 mL | 6.4047 mL | 12.8094 mL | 16.0118 mL |
| 10 mM | 0.3202 mL | 1.6012 mL | 3.2024 mL | 6.4047 mL | 8.0059 mL |
| 50 mM | 0.064 mL | 0.3202 mL | 0.6405 mL | 1.2809 mL | 1.6012 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3202 mL | 0.6405 mL | 0.8006 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (8-Acetoxy-6-methyl-3,9-dimethylene-2-oxo-4,6a,7,8,9a,9b-hexahydro-3aH-azuleno[4,5-b]furan-4-yl) 3-acetoxy-2-hydroxy-2-methyl-butanoate
Catalog No.:BCN9191
CAS No.:
- Drim-8(12)-ene-6β,7α,9α,11-tetraol
Catalog No.:BCN9190
CAS No.:2193060-24-9
- Shiraiachrome A
Catalog No.:BCN9189
CAS No.:124709-39-3
- Ustusol H4
Catalog No.:BCN9188
CAS No.:2193060-25-0
- Bistachybotrysin E
Catalog No.:BCN9187
CAS No.:2231761-98-9
- Aspergillon A
Catalog No.:BCN9186
CAS No.:2239299-08-0
- Stachartone A
Catalog No.:BCN9185
CAS No.:2209109-64-6
- Ectoine
Catalog No.:BCN9184
CAS No.:96702-03-3
- (+)-Secoisolariciresinoldiglucoside
Catalog No.:BCN9183
CAS No.:257930-74-8
- Bisisorhapontigenin G
Catalog No.:BCN9182
CAS No.:
- 2α-Acetoxy-14,15-cyclopimara-7β,16-diol
Catalog No.:BCN9181
CAS No.:2011034-27-6
- 4′-O-Isobutyroylpeguangxienin
Catalog No.:BCN9180
CAS No.:2188162-95-8
- Aflavazole
Catalog No.:BCN9193
CAS No.:2043963-70-6
- Filicane-3β,4α,25-triol
Catalog No.:BCN9194
CAS No.:2361548-00-5
- Euglobal Ia1
Catalog No.:BCN9195
CAS No.:77844-93-0
- Herbarin
Catalog No.:BCN9196
CAS No.:36379-67-6
- Dehydroherbarin
Catalog No.:BCN9197
CAS No.:36379-74-5
- 7β-Galloyloxysweroside
Catalog No.:BCN9198
CAS No.:2222365-75-3
- 5-Hydroxymethyl-2-furoic acid
Catalog No.:BCN9199
CAS No.:6338-41-6
- (Z)-ethyl cinnamate
Catalog No.:BCN9200
CAS No.:4192-77-2
- 12-Hydroxyalbrassitriol
Catalog No.:BCN9201
CAS No.:2193060-23-8
- Viburnumoside
Catalog No.:BCN9202
CAS No.:2222365-74-2
- 7α-Galloyloxysweroside
Catalog No.:BCN9203
CAS No.:2222365-76-4
- (+)-Rutamarin
Catalog No.:BCN9204
CAS No.:13164-05-1
beta-Diglycosidases from microorganisms as industrial biocatalysts: biochemical characteristics and potential applications.[Pubmed:30116842]
Appl Microbiol Biotechnol. 2018 Oct;102(20):8717-8723.
Flavonoid glycoside degradation can proceed through two alternative enzymatic pathways: one that is mediated by monoglycosidases, and the other catalyzed by a diglycosidase. beta-Diglycosidase performs the flavonoid deglycosylation in a single reaction. The characterized beta-diglycosidase activities recognize the following disaccharidic sugar moieties: beta-Primeverose, acuminose, vicianose, and beta-rutinose. The present paper reviews the biochemical characteristics and potential industrial applications of microbial beta-diglycosidases that break down plant diglycoconjugated flavonoids.
Crystal structures of beta-primeverosidase in complex with disaccharide amidine inhibitors.[Pubmed:24753293]
J Biol Chem. 2014 Jun 13;289(24):16826-34.
beta-Primeverosidase (PD) is a disaccharide-specific beta-glycosidase in tea leaves. This enzyme is involved in aroma formation during the manufacturing process of oolong tea and black tea. PD hydrolyzes beta-primeveroside (6-O-beta-d-xylopyranosyl-beta-d-glucopyranoside) at the beta-glycosidic bond of Primeverose to aglycone, and releases aromatic alcoholic volatiles of aglycones. PD only accepts Primeverose as the glycone substrate, but broadly accepts various aglycones, including 2-phenylethanol, benzyl alcohol, linalool, and geraniol. We determined the crystal structure of PD complexes using highly specific disaccharide amidine inhibitors, N-beta-primeverosylamidines, and revealed the architecture of the active site responsible for substrate specificity. We identified three subsites in the active site: subsite -2 specific for 6-O-beta-d-xylopyranosyl, subsite -1 well conserved among beta-glucosidases and specific for beta-d-glucopyranosyl, and wide subsite +1 for hydrophobic aglycone. Glu-470, Ser-473, and Gln-477 act as the specific hydrogen bond donors for 6-O-beta-d-xylopyranosyl in subsite -2. On the other hand, subsite +1 was a large hydrophobic cavity that accommodates various aromatic aglycones. Compared with aglycone-specific beta-glucosidases of the glycoside hydrolase family 1, PD lacks the Trp crucial for aglycone recognition, and the resultant large cavity accepts aglycone and 6-O-beta-d-xylopyranosyl together. PD recognizes the beta-primeverosides in subsites -1 and -2 by hydrogen bonds, whereas the large subsite +1 loosely accommodates various aglycones. The glycone-specific activity of PD for broad aglycone substrates results in selective and multiple release of temporally stored alcoholic volatile aglycones of beta-primeveroside.
Expression and biochemical characterization of beta-primeverosidase and application of beta-primeverosylamidine to affinity purification.[Pubmed:18256510]
Biosci Biotechnol Biochem. 2008 Feb;72(2):376-83.
Beta-primeverosidase (PD) is a family 1 glycosidase catalyzing the hydrolysis of beta-primeverosides (6-O-beta-D-xylopyranosyl-beta-D-glucopyranosides) to release a disaccharide Primeverose. To investigate how PD recognizes the disaccharide moiety of beta-primeverosides, the recombinant PD was expressed by a baculovirus-insect cell system. The recombinant PD was secreted from High Five cells and was properly modified with N-glycosylation and correct cleavage at the N-terminal signal peptide. The recombinant PD exhibited high substrate specificity to beta-primeverosides in terms of the glycone moiety, consistently with the substrate specificity of native PD from Camellia sinensis. Next, beta-glycosylamidines were synthesized as substrate analog inhibitors. Beta-primeverosylamidine strongly inhibited PD activity, but beta-glucosylamidine did not. Hence beta-primeverosylamidine is an ideal chemical tool for probing disaccharide recognition in the active site of PD. An affinity adsorbent for PD was prepared using beta-primeverosylamidine as a ligand. Affinity chromatography gave large amounts of PD with high purity, permitting crystallographic study.
Characteristic fragmentation patterns of the trimethylsilyl and trimethylsilyl-oxime derivatives of various saccharides as obtained by gas chromatography coupled to ion-trap mass spectrometry.[Pubmed:18061601]
J Chromatogr A. 2008 Jan 4;1177(1):183-9.
The fragmentation patterns of selected glycosidic linkage containing non-reducing (methylmannoside, methylgalactoside, lactitol, sucrose, trehalose, raffinose, erlose, melezitose) and reducing saccharides (maltose, cellobiose, lactose, melibiose, palatinose, Primeverose, rutinose) have been compared as their trimethylsilyl and as their trimethylsilyl-oxime derivatives. Fragmentation characteristics of the glycosidic linkage containing trimethylsilyl-oxime derivatives have been investigated at the first time: these spectra are not available in the official libraries (NIST, Wiley). Applying gas chromatography-ion trap mass spectrometry, informative fragments of high masses with high intensities have been obtained. Results confirmed characteristic differences between the simple trimethylsilyl derivative providing non-reducing glycosides and the trimethylsilyl, syn and antioxime species. Fragmentation starts at the glycosidic linkage resulting in the case of the non-reducing di- and trisaccharides in two, identical fragments of ring structure, with the abundant selective fragment ion at m/z=361. In the case of reducing disaccharides fragmentation provides two different moieties: one moiety of ring structure at m/z=361, and one of the open chain trimethylsilyl-oxime moiety with two special fragment ions at m/z=361 and at m/z=538. These fragmentation patterns proved to be independent on the position of the glycosidic linkage. Distribution of the selective fragment ions, obtained from their total ion current elutions, was evaluated on a quantitative basis, expressed in percentages of the total of ions formed. Reproducibility in the formation of these selective fragment ions, depending on their amount to be fragmented, proved to be proper for identification and quantitation purposes, equally. On this basis, in addition to the authentic ones, also two reducing disaccharides (Primeverose and rutinose), as authentic compounds not available on the market, were identified and quantified in natural matrices.
Isolation and characterization of a beta-primeverosidase-like enzyme from Penicillium multicolor.[Pubmed:16556987]
Biosci Biotechnol Biochem. 2006 Mar;70(3):691-8.
p-Nitrophenyl and eugenyl beta-primeveroside (6-O-beta-D-xylopyranosyl-beta-D-glucopyranoside) hydrolytic activity was found in culture filtrate from Penicillium multicolor IAM7153, and the enzyme was isolated. The enzyme was purified as a beta-primeverosidase-like enzyme by precipitation with ammonium sulfate followed by successive chromatographies on Phenyl Sepharose, Mono Q, and beta-galactosylamidine affinity columns. The molecular mass was estimated to be 50 kDa by SDS-PAGE and gel filtration. The purified enzyme was highly specific toward the substrate p-nitrophenyl beta-primeveroside, which was cleaved in an endo-manner into Primeverose and p-nitrophenol, but a series of beta-primeveroside as aroma precursors were hydrolyzed only slightly as substrates for the enzyme. In analyses of its hydrolytic action and kinetics, the enzyme showed narrow substrate specificity with respect to the aglycon and glycon moieties of the diglycoside. We conclude that the present enzyme is a kind of beta-diglycosidase rather than beta-primeverosidase.
Cloning of beta-primeverosidase from tea leaves, a key enzyme in tea aroma formation.[Pubmed:12481100]
Plant Physiol. 2002 Dec;130(4):2164-76.
A beta-primeverosidase from tea (Camellia sinensis) plants is a unique disaccharide-specific glycosidase, which hydrolyzes aroma precursors of beta-primeverosides (6-O-beta-D-xylopyranosyl-beta-D-glucopyranosides) to liberate various aroma compounds, and the enzyme is deeply concerned with the floral aroma formation in oolong tea and black tea during the manufacturing process. The beta-primeverosidase was purified from fresh leaves of a cultivar for green tea (C. sinensis var sinensis cv Yabukita), and its partial amino acid sequences were determined. The beta-primeverosidase cDNA has been isolated from a cDNA library of cv Yabukita using degenerate oligonucleotide primers. The cDNA insert encodes a polypeptide consisting of an N-terminal signal peptide of 28 amino acid residues and a 479-amino acid mature protein. The beta-primeverosidase protein sequence was 50% to 60% identical to beta-glucosidases from various plants and was classified in a family 1 glycosyl hydrolase. The mature form of the beta-primeverosidase expressed in Escherichia coli was able to hydrolyze beta-primeverosides to liberate a Primeverose unit and aglycons, but did not act on 2-phenylethyl beta-D-glucopyranoside. These results indicate that the beta-primeverosidase selectively recognizes the beta-primeverosides as substrates and specifically hydrolyzes the beta-glycosidic bond between the disaccharide and the aglycons. The stereochemistry for enzymatic hydrolysis of 2-phenylethyl beta-primeveroside by the beta-primeverosidase was followed by (1)H-nuclear magnetic resonance spectroscopy, revealing that the enzyme hydrolyzes the beta-primeveroside by a retaining mechanism. The roles of the beta-primeverosidase in the defense mechanism in tea plants and the floral aroma formation during tea manufacturing process are also discussed.
Isolation and characterization of a beta-primeverosidase-like endo-manner beta-glycosidase from Aspergillus fumigatus AP-20.[Pubmed:12036053]
Biosci Biotechnol Biochem. 2002 Apr;66(4):801-7.
A novel beta-glycosidase-producing microorganism was isolated from soil and identified as Aspergillus fumigatus AP-20 based on its taxonomical characteristics. The enzyme was found to be an extracellular protein in the culture of the isolated fungus and was purified 88-fold by fractionation with ammonium sulfate followed by successive column chromatographies on phenyl-Sepharose HP and Mono P HR. The molecular mass was estimated to be 47 kDa by SDS-PAGE and the isoelectric point to be pH 6.0 by isoelectric focusing. The purified enzyme was highly specific for a substrate, p-nitrophenyl beta-primeveroside (6-O-beta-D-xylopyranosyl-beta-D-glucopyranoside), which was cleaved in an endo-manner into Primeverose and p-nitrophenol.
Enzymatic synthesis and characterization of 6-O-Beta-D-xylopyranosyl-2-acetamido-2-deoxy-D-glucopyranose, a structural analog of primeverose.[Pubmed:9821268]
Carbohydr Res. 1998 Sep;311(1-2):79-83.
The synthesis of the disaccharide 6-O-beta-D-xylopyranosyl-2-acetamido-2-deoxy-D-glucopyranose (N-acetylprimeverosamine), structurally related to the natural disaccharide 6-O-beta-D-xylopyranosyl-D-glycopyranose (Primeverose), was obtained via a transglycosylation reaction catalyzed by a crude preparation of beta-D-xylosidase from Aspergillus niger, using p-nitrophenyl beta-D-xylopyranoside as the donor and 2-acetamido-2-deoxy-D-glucopyranose as the acceptor. The yield of the reaction was 36% on a molar basis with respect to the donor. The chemical identity of the product was assessed by HPLC, ionspray mass spectrometry and NMR spectroscopy.


