DehydroherbarinCAS# 36379-74-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36379-74-5 | SDF | Download SDF |
| PubChem ID | 11833010 | Appearance | Red powder |
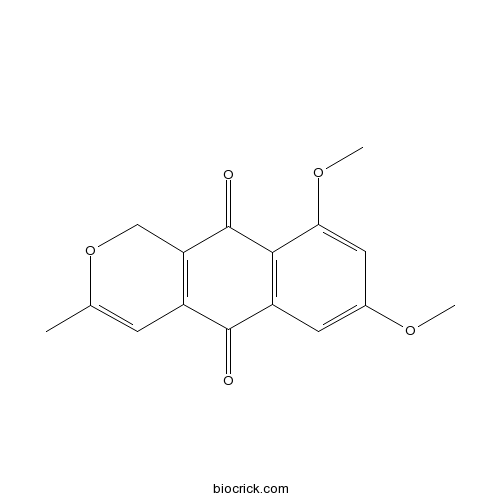
| Formula | C16H14O5 | M.Wt | 286.28 |
| Type of Compound | Other Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7,9-dimethoxy-3-methyl-1H-benzo[g]isochromene-5,10-dione | ||
| SMILES | CC1=CC2=C(CO1)C(=O)C3=C(C2=O)C=C(C=C3OC)OC | ||
| Standard InChIKey | ITUSOEBAIVROCD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O5/c1-8-4-10-12(7-21-8)16(18)14-11(15(10)17)5-9(19-2)6-13(14)20-3/h4-6H,7H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dehydroherbarin Dilution Calculator

Dehydroherbarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4931 mL | 17.4654 mL | 34.9308 mL | 69.8617 mL | 87.3271 mL |
| 5 mM | 0.6986 mL | 3.4931 mL | 6.9862 mL | 13.9723 mL | 17.4654 mL |
| 10 mM | 0.3493 mL | 1.7465 mL | 3.4931 mL | 6.9862 mL | 8.7327 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6986 mL | 1.3972 mL | 1.7465 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3493 mL | 0.6986 mL | 0.8733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Herbarin
Catalog No.:BCN9196
CAS No.:36379-67-6
- Euglobal Ia1
Catalog No.:BCN9195
CAS No.:77844-93-0
- Filicane-3β,4α,25-triol
Catalog No.:BCN9194
CAS No.:2361548-00-5
- Aflavazole
Catalog No.:BCN9193
CAS No.:2043963-70-6
- Primeverose
Catalog No.:BCN9192
CAS No.:26531-85-1
- (8-Acetoxy-6-methyl-3,9-dimethylene-2-oxo-4,6a,7,8,9a,9b-hexahydro-3aH-azuleno[4,5-b]furan-4-yl) 3-acetoxy-2-hydroxy-2-methyl-butanoate
Catalog No.:BCN9191
CAS No.:
- Drim-8(12)-ene-6β,7α,9α,11-tetraol
Catalog No.:BCN9190
CAS No.:2193060-24-9
- Shiraiachrome A
Catalog No.:BCN9189
CAS No.:124709-39-3
- Ustusol H4
Catalog No.:BCN9188
CAS No.:2193060-25-0
- Bistachybotrysin E
Catalog No.:BCN9187
CAS No.:2231761-98-9
- Aspergillon A
Catalog No.:BCN9186
CAS No.:2239299-08-0
- Stachartone A
Catalog No.:BCN9185
CAS No.:2209109-64-6
- 7β-Galloyloxysweroside
Catalog No.:BCN9198
CAS No.:2222365-75-3
- 5-Hydroxymethyl-2-furoic acid
Catalog No.:BCN9199
CAS No.:6338-41-6
- (Z)-ethyl cinnamate
Catalog No.:BCN9200
CAS No.:4192-77-2
- 12-Hydroxyalbrassitriol
Catalog No.:BCN9201
CAS No.:2193060-23-8
- Viburnumoside
Catalog No.:BCN9202
CAS No.:2222365-74-2
- 7α-Galloyloxysweroside
Catalog No.:BCN9203
CAS No.:2222365-76-4
- (+)-Rutamarin
Catalog No.:BCN9204
CAS No.:13164-05-1
- 3,3-Dimethylacrylic acid
Catalog No.:BCN9205
CAS No.:541-47-9
- O-Demethylstachartin C
Catalog No.:BCN9206
CAS No.:1219361-60-0
- Qingyangshengenin 3-O-α-L-cymaropyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside
Catalog No.:BCN9207
CAS No.:1808159-02-5
- Chimaphilin
Catalog No.:BCN9208
CAS No.:482-70-2
- Ethyl chlorogenate
Catalog No.:BCN9209
CAS No.:425408-42-0
Modulation of polyketide biosynthetic pathway of the endophytic fungus, Anteaglonium sp. FL0768, by copper (II) and anacardic acid.[Pubmed:31354886]
Phytochem Lett. 2018 Dec;28:157-163.
In an attempt to explore the biosynthetic potential of endosymbiotic fungi, the secondary metabolite profiles of the endophytic fungus, Anteaglonium sp. FL0768, cultured under a variety of conditions were investigated. In potato dextrose broth (PDB) medium, Anteaglonium sp. FL0768 produced the heptaketides, herbaridine A (1), herbarin (2), 1-hydroxyDehydroherbarin (3), scorpinone (4), and the methylated hexaketide 9S,11R-(+)-ascosalitoxin (5). Incorporation of commonly used epigenetic modifiers, 5-azacytidine and suberoylanilide hydroxamic acid, into the PDB culture medium of this fungus had no effect on its secondary metabolite profile. However, the histone acetyl transferase inhibitor, anacardic acid, slightly affected the metabolite profile affording scorpinone (4) as the major metabolite together with 1-hydroxyDehydroherbarin (3) and a different methylated hexaketide, ascochitine (6). Intriguingly, incorporaion of Cu(2+) into the PDB medium enhanced production of metabolites and drastically affected the biosynthetic pathway resulting in the production of pentaketide dimers, palmarumycin CE4 (7), palmarumycin CP4 (8), and palmarumycin CP1 (9), in addition to ascochitine (6). The structure of the new metabolite 7 was established with the help of spectroscopic data and by MnO2 oxidation to the known pentaketide dimer, palmarumycin CP3 (10). Biosynthetic pathways to some metabolites in Anteaglonium sp. FL0768 are presented and possible effects of AA and Cu(2+) on these pathways are discussed.
Applications of [4+2] Anionic Annulation and Carbonyl-Ene Reaction in the Synthesis of Anthraquinones, Tetrahydroanthraquinones, and Pyranonaphthoquinones.[Pubmed:28959889]
J Org Chem. 2017 Oct 20;82(20):11035-11051.
Hexa-2,5-dienoates, susceptible to isomerization by acids and bases, are suitable for the [4+2] anionic annulation to give 3-(2-alkenyl)naphthoates in regiospecific manner. When combined with intramolecular carbonyl-ene reaction (ICE), the accessibility of the naphthoates culminates in a new synthesis of anthraquinones and diastereoselective synthesis of tetrahydroanthraquinones. This strategy has also resulted in a 3-step synthesis of Dehydroherbarin from a 3-methallylnaphthoate.
The synthesis of the pyranonaphthoquinones dehydroherbarin and anhydrofusarubin using Wacker oxidation methodology as a key step and other unexpected oxidation reactions with ceric ammonium nitrate and salcomine.[Pubmed:22915091]
Org Biomol Chem. 2012 Oct 14;10(38):7809-19.
The synthesis of two closely related pyranonaphthoquinones, Dehydroherbarin and anhydrofusarubin, is described. The construction of the naphthalene nuclei was achieved using the Stobbe condensation reaction using 2,4-dimethoxybenzaldehyde and 2,4,5-trimethoxybenzaldehyde as their respective starting materials. Two key steps en route include a PIFA-mediated addition of a methoxy substituent onto the naphthalene skeleton and a Wacker oxidation reaction to construct the benzo[g]isochromene nucleus. Two interesting oxidation reactions of the intermediate isochromene enol ether of 7,9-dimethoxy-3-methyl-1H-benzo[g]isochromene-5-ol were observed. Treatment of the substrate with salcomine resulted in the formation of (3-formyl-4-hydroxy-6,8-dimethoxynaphthalene-2-yl)methyl acetate, while treatment of the same substrate with CAN resulted in the formation of racemic (3R,4R)-3-hydroxy-7,9-dimethoxy-3-methyl-5,10-dioxo-3,4,5,10-tetrahydro-1H-benzo[ g]isochromen-4-yl nitrate.
Maximizing chemical diversity of fungal metabolites: biogenetically related Heptaketides of the endolichenic fungus Corynespora sp. (1).[Pubmed:20521776]
J Nat Prod. 2010 Jun 25;73(6):1156-9.
In an attempt to explore the biosynthetic potential of the endolichenic fungus Corynespora sp. BA-10763, its metabolite profiles under several culture conditions were investigated. When cultured in potato dextrose agar, it produced three new heptaketides, 9-O-methylscytalol A (1), 7-desmethylherbarin (2), and 8-hydroxyherbarin (3), together with biogenetically related metabolites scytalol A (4), 8-O-methylfusarubin (5), scorpinone (6), and 8-O-methylbostrycoidin (7), which are new to this organism, and herbarin (8), a metabolite previously encountered in this fungal strain. The use of malt extract agar as the culture medium led to the isolation of 6, 8, 1-hydroxyDehydroherbarin (9), and 1-methoxyDehydroherbarin (10), which was found to be an artifact formed during the extraction of the culture medium with methanol. The structures of all new compounds were determined by interpretation of their spectroscopic data and chemical interconversions.
Synthesis of highly functionalized bis(4H-chromene) and 4H-benzo[g]chromene derivatives via an isocyanide-based pseudo-five-component reaction.[Pubmed:19397302]
J Org Chem. 2009 Jun 5;74(11):4372-4.
The reactive intermediates generated by the addition of alkyl, aryl, and alicyclic isocyanides to dialkyl acetylenedicarboxylates were trapped by 2,5-dihydroxycyclohexa-2,5-diene-1,4-dione or 2-hydroxynaphthalene-1,4-dione to produce highly functionalized bis(4H-chromene)- and 4H-benzo[g]chromene-3,4-dicarboxylate derivatives in fairly good yields in CH(3)CN at room temperature. These compounds are closely related to the ring systems pentalongin, Dehydroherbarin, 1,3-disubstituted-3,4-dehydropyranonaphthoquinones, and 3-arylpyranonaphthoquinones, which have a broad spectrum of biological activity.
New naphthoquinone derivatives from the ascomycete IBWF79B-90A.[Pubmed:19323262]
Z Naturforsch C J Biosci. 2009 Jan-Feb;64(1-2):25-31.
Bioactivity-guided fractionation of extracts from the fungus IBWF79B-90A resulted in the isolation of three known naphthoquinones, herbarin, Dehydroherbarin, and O-methylherbarin and the azaanthraquinone scorpinone as well as three structurally related derivatives, O-phenethylherbarin and herbaridines A and B. All seven compounds exhibited cytotoxic activities against several cell lines.
Heptaketides from Corynespora sp. inhabiting the cavern beard lichen, Usnea cavernosa: first report of metabolites of an endolichenic fungus.[Pubmed:17988097]
J Nat Prod. 2007 Nov;70(11):1700-5.
Two new heptaketides, corynesporol (1) and 1-hydroxyDehydroherbarin (2), along with herbarin (3) were isolated from an endolichenic fungal strain, Corynespora sp. BA-10763, occurring in the cavern beard lichen Usnea cavernosa. The structures of 1-3 were elucidated from their spectroscopic data. Aerial oxidation of corynesporol (1) yielded herbarin (3). Acetylation of 1 afforded the naphthalene derivative 4, whereas acetylation of 3 gave the corresponding naphthoquinone 6 and Dehydroherbarin (5). All compounds were evaluated for their cytotoxicity and ability to inhibit migration of human metastatic breast and prostate cancer cell lines MDA-MB-231 and PC-3M, respectively. Dehydroherbarin (5) inhibited migration of both cell lines at concentrations not toxic to these cell lines. This is the first report of metabolites from an endolichenic fungus.
Synthesis of two naphthoquinone antibiotics, dehydroherbarin and 6-deoxybostrycoidin.[Pubmed:10813991]
J Org Chem. 2000 Feb 11;65(3):640-4.
The synthesis of two naphthoquinone antibiotics, Dehydroherbarin (7) and 6-deoxybostrycoidin (5), was accomplished by reaction of 3-acetonyl-2-bromomethyl-6,8-dimethoxy-1,4-naphthoquinone (23) with either triethylamine or ammonia, respectively. This is the first report on their synthesis.


