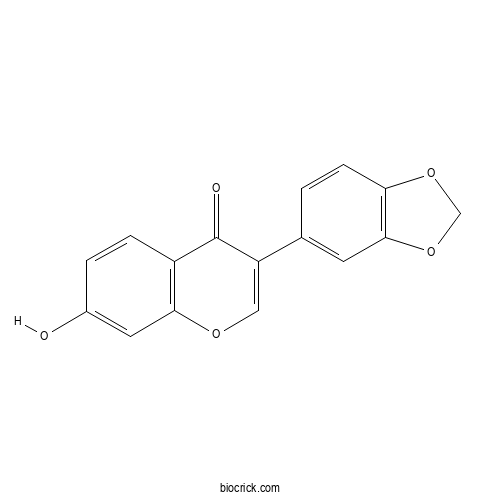
PseudobaptigeninCAS# 90-29-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90-29-9 | SDF | Download SDF |
| PubChem ID | 5281805 | Appearance | Powder |
| Formula | C16H10O5 | M.Wt | 282.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 7-Hydroxy-3',4'-methylenedioxyisoflavone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(1,3-benzodioxol-5-yl)-7-hydroxychromen-4-one | ||
| SMILES | C1OC2=C(O1)C=C(C=C2)C3=COC4=C(C3=O)C=CC(=C4)O | ||
| Standard InChIKey | KNJNBKINYHZUGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H10O5/c17-10-2-3-11-14(6-10)19-7-12(16(11)18)9-1-4-13-15(5-9)21-8-20-13/h1-7,17H,8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pseudobaptigenin Dilution Calculator

Pseudobaptigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5436 mL | 17.7179 mL | 35.4359 mL | 70.8717 mL | 88.5897 mL |
| 5 mM | 0.7087 mL | 3.5436 mL | 7.0872 mL | 14.1743 mL | 17.7179 mL |
| 10 mM | 0.3544 mL | 1.7718 mL | 3.5436 mL | 7.0872 mL | 8.859 mL |
| 50 mM | 0.0709 mL | 0.3544 mL | 0.7087 mL | 1.4174 mL | 1.7718 mL |
| 100 mM | 0.0354 mL | 0.1772 mL | 0.3544 mL | 0.7087 mL | 0.8859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-Oxostenine
Catalog No.:BCN0403
CAS No.:18058-98-5
- 7-Prenyloxyaromadendrin
Catalog No.:BCN0402
CAS No.:78876-50-3
- 2'-Prenylsemilicoisoflavone B
Catalog No.:BCN0401
CAS No.:651750-10-6
- Muurol-4-ene-3,8-dione
Catalog No.:BCN0400
CAS No.:105181-07-5
- Gnetucleistol F
Catalog No.:BCN0399
CAS No.:913529-99-4
- 9α-Hydroxymatrine
Catalog No.:BCN0398
CAS No.:88509-92-6
- 9α-Hydroxysophocarpine
Catalog No.:BCN0397
CAS No.:907607-58-3
- Poilaneic acid
Catalog No.:BCN0396
CAS No.:80489-67-4
- Lehmbachol C
Catalog No.:BCN0395
CAS No.:189295-08-7
- Genkwanoid B
Catalog No.:BCN0394
CAS No.:2374149-39-8
- 2',4',6'-Trihydroxydihydrochalcone 4'-O-(3''-O-galloyl)glucoside
Catalog No.:BCN0393
CAS No.:1885097-49-3
- 4-Deoxyphlorizin
Catalog No.:BCN0392
CAS No.:31018-48-1
- Tuberostemonine A
Catalog No.:BCN0405
CAS No.:876313-35-8
- Tuberospironine A
Catalog No.:BCN0406
CAS No.:1417863-77-4
- Flavinantine
Catalog No.:BCN0407
CAS No.:19777-82-3
- Elasine
Catalog No.:BCN0408
CAS No.:123064-65-3
- 6β,8-Dihydroxyeremophil-7(11)-en-12,8-olide
Catalog No.:BCN0409
CAS No.:58848-38-7
- Siwanine A
Catalog No.:BCN0410
CAS No.:159903-64-7
- erythro-Guaiacylglycerol β-coniferyl ether
Catalog No.:BCN0411
CAS No.:168252-52-6
- 6β-Ethoxy-8β,10β-dihydroxyeremophil-7(11)-en-12,8α-olide
Catalog No.:BCN0412
CAS No.:2260801-15-6
- 3',4-Dihydroxy-3,5'-dimethoxybibenzyl
Catalog No.:BCN0413
CAS No.:83088-28-2
- Cryptomerin B
Catalog No.:BCN0414
CAS No.:22012-98-2
- Euphorbia factor L1
Catalog No.:BCN0415
CAS No.:76376-43-7
- Bisandrographolide C
Catalog No.:BCN0416
CAS No.:160498-02-2
Investigation of red clover (Trifolium pratense) Isoflavonoid residual complexity by off-line CCS-qHNMR.[Pubmed:34416305]
Fitoterapia. 2021 Aug 17:105016.
The importance of Trifolium pratense L. as a dietary supplement and its use in traditional medicine prompted the preparation of a thorough metabolite profile. This included the identification and quantitation of principal constituents as well as low abundant metabolites that constitute the residual complexity (RC) of T. pratense bioactives. The purity and RC of isoflavonoid fractions from standardized red clover extract (RCE) was determined using an off-line combination of countercurrent separation (CCS) and two orthogonal analytical methodologies: quantitative (1)H NMR spectroscopy with external calibration (EC-qHNMR) and LC-MS. A single-step hydrostatic CCS methodology (Centrifugal Partition Chromatography [CPC]) was developed that fractionated the isoflavonoids with a hexanes-ethyl acetate-methanol-water (HEMWat) 5.5/4.5/5/5, v/v solvent system (SS) into 75 fractions containing 3 flavonolignans, 2 isoflavonoid glycosides, as well as 17 isoflavonoids and related compounds. All metabolites were identified and quantified by qHNMR spectroscopy. The data led to the creation of a complete isoflavonoid profile to complement the biological evaluation. For example, fraction 69 afforded 90.5% w/w biochanin A (17), with 0.33% w/w of prunetin (16), and 0.76% w/w of maackiain (15) as residual components. Fraction 27 with 89.4% w/w formononetin (13) as the major component had, in addition, a residual complexity consisting of 3.37%, 0.73%, 0.68% w/w of Pseudobaptigenin (11), kaempferol (10) and pratensein (8), respectively. Despite the relatively high resolving power of CPC, and not unexpectedly, the chromatographic fractions retained varying degrees of the original metabolomic diversity. Collectively, the extent of metabolomic diversity should be recognized and used to guide the development of isolation strategies, especially when generating samples for bioactivity evaluation. The simultaneous structural and quantitative characterization enabled by qNMR, supported by LC-MS measurements, enables the evaluation of a relatively large number of individual fractions and, thereby, advances both the chemical and biological evaluation of active principles in complex natural products.
Preparation of DESIGNER extracts of red clover (Trifolium pratense L.) by centrifugal partition chromatography.[Pubmed:31307793]
J Chromatogr A. 2019 Nov 8;1605:360277.
Starting with an isoflavone-rich red clover extract (RCE), this study expands on the DESIGNER approach to Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources using countercurrent separation (CCS) methodology. A hydrostatic CCS (also known as centrifugal partition chromatography, CPC) technique was used to enrich and deplete selected bioactive isoflavones of RCE extracts. In order to efficiently prepare large enough DESIGNER extracts from RCE for biological testing including in vivo assays, it was necessary to choose a balance between resolution and a loading capacity of at least 1g per separation for the selected solvent system (SS). Adding 3mL of DMSO to the sample containing equal amounts of upper and lower phases of hexanes-ethyl acetate-methanol-water (HEMWat 5.5/4.5/5/5, v/v) allowed 1g of RCE to be dissolved in the sample without disrupting the chromatographic resolution of the target isoflavones. CPC experiments using other solubility modifiers, acetone and acetonitrile indicated that these modifiers increase solubility significantly, even better than DMSO, but the separation of target compounds was sufficiently disturbed to be unacceptable for producing the desired DESIGNER extracts. The preparation of DESIGNER extracts was achieved with two sequential CPC separations. The first produced a biochanin A enriched fraction (93.60% w/w) with only small amounts of other isoflavones: 2.30% w/w prunetin, 1.17% w/w formononetin, and 0.12% w/w irilone. Gravimetric investigations of this step demonstrated the high efficiency of CCS technology for full and unbiased sample recovery, confirmed experimentally to be 99.80%. A formononetin enriched fraction from this first separation was re-chromatographed on a more polar HEMWat (4/6/4/6, v/v) SS to produce a formononetin enriched DESIGNER fraction of 94.70% w/w purity. The presence of the minor (iso)flavonoids: 3.16% w/w Pseudobaptigenin, 0.39% w/w kaempferol, and 0.31% w/w genistein was also monitored in these fractions. Chromatographic fractions, combined fractions, and DESIGNER extracts were analyzed with quantitative (1)H NMR (qHNMR) spectroscopy which provided purity information, quantitation, and structural identification of the components.
Screening and isolation of cyclooxygenase-2 inhibitors from Trifolium pratense L. via ultrafiltration, enzyme-immobilized magnetic beads, semi-preparative high-performance liquid chromatography and high-speed counter-current chromatography.[Pubmed:30620132]
J Sep Sci. 2019 Mar;42(6):1133-1143.
Nonsteroidal anti-inflammatory drugs reportedly reduce the risk of developing cancer. One mechanism by which they reduce carcinogenesis involves the inhibition of the activity of cyclooxygenase-2, an enzyme that is overexpressed in various cancer tissues. Its overexpression increases cell proliferation and inhibits apoptosis. However, selected cyclooxygenase-2 inhibitors can also act through cyclooxygenase-independent mechanisms. In this study, using ultrafiltration, enzyme-immobilized magnetic beads, high-performance liquid chromatography, and electrospray-ionization mass spectrometry, several isoflavonoids in Trifolium pratense L. extracts were screened and identified. Semi-preparative high-performance liquid chromatography and high-speed counter-current chromatography were then applied to separate the active constituents. Using these methods, seven major compounds were identified in Trifolium pratense L. As cyclooxygenase-2 inhibitors: rothindin, ononin, daidzein, trifoside, Pseudobaptigenin, formononetin, and biochanin A, which were then isolated with >92% purity. This is the first report of the presence of potent cyclooxygenase-2 inhibitors in Trifolium pratense L. extracts. The results of this study demonstrate that the systematic isolation of bioactive components from Trifolium pratense L., by using ultrafiltration, enzyme-immobilized magnetic beads, semi-preparative high-performance liquid chromatography, and high-speed counter-current chromatography, represents a feasible and efficient technique that could be extended for the identification and isolation of other enzyme inhibitors.
Identification of Hypotensive Biofunctional Compounds of Coriandrum sativum and Evaluation of Their Angiotensin-Converting Enzyme (ACE) Inhibition Potential.[Pubmed:30581531]
Oxid Med Cell Longev. 2018 Nov 15;2018:4643736.
The aim of this study was to identify and characterize the bioactive compounds of Coriandrum sativum responsible for the treatment of hypertension and to explore their mechanism of action as angiotensin-converting enzyme (ACE) inhibitors. Bioactive fractions like alkaloids, flavonoids, steroids, and tannins were extracted and evaluated for their ACE inhibition potential. Among them, only flavonoid-rich fraction showed high ACE inhibition potential with IC50 value of 28.91 +/- 13.42 mug/mL. The flavonoids were characterized through LC-ESI-MS/MS. Seventeen flavonoids were identified in this fraction of Coriandrum sativum in negative ionization mode which includes pinocembrin, apigenin, Pseudobaptigenin, galangin-5-methyl ether, quercetin, baicalein trimethyl ether, kaempferol dimethyl ether, pinobanksin-5-methylether-3-O-acetate, pinobanksin-3-O-pentenoate, pinobanksin-3-O-phenylpropionate, pinobanksin-3-O-pentanoate, apigenin-7-O-glucuronoide, quercetin-3-O-glucoside, apigenin-3-O-rutinoside, rutin, isorhamnetin-3-O-rutinoside, and quercetin dimethyl ether-3-O-rutinoside, while six flavonoids including daidzein, luteolin, pectolinarigenin, apigenin-C-glucoside, kaempferol-3-7-dimethyl ether-3-O-glucoside, and apigenin-7-O-(6-methyl-beta-D-glucoside) were identified in positive ionization mode. The results of this study revealed that Coriandrum sativum is a valuable functional food that possesses a number of therapeutic flavonoids with ACE inhibition potential that can manage blood pressure very efficiently.
Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root.[Pubmed:29803686]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Aug 1;1091:21-28.
Spiny restharrow root (Ononis spinosa L.) and its preparations are mainly used for the treatment of urinary infections or bladder stones in numerous countries. Spiny restharrow root is rich in isoflavonoids (formononetin, calycosin and Pseudobaptigenin), pterocarpans (medicarpin and maackiain) and dihydroisoflavonoids (onogenin and sativanone), which metabolites are present as glucosides, glucoside malonates, glucoside acetates and free aglycones in the root. The in-depth analysis of tandem mass spectrometric (MS) and high-resolution MS (HR-MS) data revealed the presence of nitrogen-containing compounds in the root extracts. An ion-exchange-based purification and a preparative-scale reversed phase chromatographic isolation procedure was developed for the characterization of these new natural products. For the unambiguous identification of the isolated compounds NMR experiments were carried out. The thorough characterization confirmed the presence of six piperidin-2-yl-acetic acid (homopipecolic acid) esters of isoflavonoid glucosides. This is the first report of homopipecolic acid esters isolated from higher plants.
Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC-MS/MS and NMR.[Pubmed:26874257]
J Pharm Biomed Anal. 2016 May 10;123:74-81.
Restharrow root has been used in traditional medicine for thousands of years; however, the active ingredients responsible for the diuretic effect are still unknown. Previous studies have proved that the root extract contains isoflavonoids, however only few derivatives were identified, mostly relying on retention times or UV data. The aim of our work was to perform a detailed structural characterization of the complete isoflavonoid profile in the aqueous-methanolic extract of Ononis spinosa root by high-performance liquid chromatography coupled with electrospray ionization accurate-mass quadrupole time-of-flight and tandem mass spectrometry in positive ionization mode (HPLC-ESI-QTOF-MS, HPLC-ESI-MS/MS) and nuclear magnetic resonance spectroscopy (NMR). On the basis of the accurate masses and fragmentation patterns isoflavones (formononetin, calycosin and Pseudobaptigenin) and pterocarpans (maackiain and medicarpin) were identified. Two further dihydroisoflavone aglycones, namely onogenin and sativanone and a unique glucoside were isolated and their structures were elucidated by NMR experiments. Calycosin, onogenin and sativanone were detected in this plant for the first time. In contrast to previous works, the presence of biochanin A could not be confirmed, however its regioisomer calycosin and its derivatives were identified. Similarly, neither tectorigenin derivatives could be detected, however the isobar compound sativanone and its various glucosides were elucidated. The presence of genistein and daidzein could not be confirmed in the extract. Fragmentation pathways for onogenin and sativanone are presented. In the aqueous-methanolic extract 9 glucosides, 6 minor and 8 major glucoside malonates, 4 glucoside acetates and 7 aglycones were found. In total, 34 compounds were successfully identified.
Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions.[Pubmed:25630489]
J Agric Food Chem. 2015 Mar 18;63(10):2701-7.
The influence of different germination conditions on isoflavone contents in chickpea sprouts was investigated in this study. Chickpea sprouts were germinated under different experimental conditions, including germination in the dark (GD), in the light (GL), under ethanol stress (GE), or under salt stress (GS) in the dark. The results demonstrated that the isoflavone contents in chickpea sprouts germinated with these various conditions significantly increased (p<0.05) compared to those in untreated chickpea seeds. The maximum amount of total isoflavones was obtained from chickpea sprouts in the GL group on day 8. The contents of formononetin and biochanin A in this group were 154 and 130 times higher, respectively, than in untreated seeds and 1.2 times higher than in sprouts in the GD group. Moreover, the isoflavone contents of chickpea sprouts in the GE and GS groups were also higher (p<0.05) than those in the GD group. A solution of 3% ethanol and 0.03 mol/L salt seemed to be the most optimal for isoflavone production among the solutions selected for this study. Most of the isoflavone contents significantly increased (p<0.05), especially formononetin and biochanin A, while the genistein content decreased with germination. Ononin, Pseudobaptigenin, and glycitein glucoside acetylated were only detected in germinated chickpeas. This finding could expand the potential for the development of chickpea sprouts as a functional food.
Isoflavone production in Cyclopia subternata Vogel (honeybush) suspension cultures grown in shake flasks and stirred-tank bioreactor.[Pubmed:23872960]
Appl Microbiol Biotechnol. 2013 Oct;97(19):8467-77.
Suspension cultures of the endemic South-African plant Cyclopia subternata were established for the first time and evaluated for the presence of isoflavones. The influence of light, as well as medium supplementation strategies with phenylalanine, casein hydrolysate and coconut water on biomass growth and isoflavone production were examined. The highest levels of 7-O-beta-glucosides of calycosin, Pseudobaptigenin and formononetin (275.57, 125.37 and 147.28 mg/100 g DW, respectively) were recorded for cultures grown in the absence of light, whereas coconut water substantially promoted biomass growth. Cell suspensions were subsequently grown in the 2-l stirred-tank bioreactor. Maximum productivity of 7-O-beta-glucosides of calycosin, Pseudobaptigenin and formononetin (0.96, 0.44 and 0.22 mg l(-1) day(-1), respectively) in bioreactor-cultivated cells was obtained for biomass grown in the dark and supplemented with coconut water. The results indicate that C. subternata suspension cultures can be utilised for the production of the specified isoflavone derivatives absent in the intact plant.
LC-ESI-MS characterisation of phytoalexins induced in chickpea and pea tissues in response to a biotic elicitor of Hypnea musciformis (red algae).[Pubmed:21859260]
Nat Prod Res. 2011 Aug;25(14):1352-60.
A simple extraction procedure and HPLC method was developed to analyse the major and minor components of induced phytoalexins of elicited tissues (seeds) of chickpeas (Cicer arietinum L.) and peas (Pisum sativum L.) treated with a biotic elicitor (k-carrageenan) of Hypnea musciformis (red algae) from the Karachi coast. The level and timing of the induced phytoalexin production were estimated on the basis of various elicitor dilutions and as a function of time; the results are presented and discussed. A LC-ESI-MS/MS technique has been employed for the detection and characterisation of the induced phytochemical components (flavonoids and their glyco-conjugates). Nine flavonoids were identified from chickpeas: naringin, naringin malonate, liquiritigenin, naringenin, biochanin A, daidzein, formononetin, maackiain and medicarpin, while five flavonoids were identified from peas: afrormosin, anhydropisatin, pisatin, Pseudobaptigenin and maackiain. These compounds play a vital role as phytoalexins because of their antimicrobial activity.
Dalnigrin, a neoflavonoid marker for the identification of Brazilian rosewood (Dalbergia nigra) in CITES enforcement.[Pubmed:20457458]
Phytochemistry. 2010 Jul;71(10):1122-31.
International trade in Brazilian rosewood, Dalbergia nigra (Vell.) Allemao ex Benth., is regulated by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). One problem in enforcing these regulations is the difficulty in distinguishing the wood of D. nigra from that of a closely-related but unregulated species, Dalbergia spruceana Benth. Using LC-MS to analyse methanol extracts of xylaria specimens, we identified a chemical marker for D. nigra heartwood, and determined its structure as the neoflavonoid 6-hydroxy-7-methoxy-4-(4-methoxyphenyl)-2H-1-benzopyran-2-one (4'-O-methylmelanettin; dalnigrin), using spectroscopic techniques. Dalnigrin was present in all nine available heartwood specimens of D. nigra, but it was not detected in extracts of 59 other heartwood samples representing 15 species of Dalbergia, including D. spruceana. Five other phenolic compounds were also isolated from D. nigra heartwood and similarly identified as the neoflavonoids 3'-hydroxymelanettin, melanettin, melannein and dalbergin, and the isoflavone caviunin. In extracts of D. spruceana heartwood, Pseudobaptigenin was identified by LC-MS to be a major phenolic component that was not detected in wood extracts of D. nigra. We conclude that chemical analysis, in combination with anatomical investigation, can provide persuasive evidence to support the positive identification of untreated heartwood of D. nigra.
Red clover (Trifolium pratense L.) isoflavones: root phenolic compounds affected by biotic and abiotic stress factors.[Pubmed:20355062]
J Sci Food Agric. 2010 Feb;90(3):418-23.
BACKGROUND: Phenolic compounds have recently received considerable attention for their ability to protect plant and human cells from oxidative stress-induced damage. Red clover (Trifolium pratense L.) is a rich source of isoflavonoids with multiple potential protective functions. The aim of this study was to identify and characterise phenolic compounds in red clover roots by high-performance liquid chromatography and mass spectrometry and to study the effects of stress factors and growth stage on root phenolics. RESULTS: A total of 28 phenolic compounds were tentatively identified in red clover roots. The most abundant phenolics in pot-grown roots were formononetin glycoside malonate (G-M) (1.51-4.26 mg g(-1)), formononetin (2.21-3.57 mg g(-1)) and biochanin A (1.73-2.17 mg g(-1)), whereas field-grown roots were rich in formononetin-G-M (3.90-4.27 mg g(-1)), maackiain-G-M (2.35-3.02 mg g(-1)) and Pseudobaptigenin-G-M (1.80-2.58 mg g(-1)). Concentrations were affected by the growth stage. Ozone exposure slightly affected the total phenolic content in roots and also had minor effects on individual compounds. CONCLUSION: Elevated ozone, cultivation regime and growth stage affected the levels of phenolics in red clover roots, suggesting sensitivity of root phenolics to biotic and abiotic stress conditions. The high levels of phenolics found in roots even in late autumn may be utilised in many applications.
Absorption of red clover isoflavones in human subjects: results from a pilot study.[Pubmed:20067656]
Br J Nutr. 2010 Jun;103(11):1569-72.
In addition to soya-derived preparations, red clover-based dietary supplements have gained considerable interest as an alternative isoflavone (IF) source. While metabolism and bioavailability of the main IF from both sources have already been investigated, studies are still lacking on the biokinetic behaviour of IF, which are present in red clover in minor amounts. In the present pilot study, in which seven volunteers ingested a single dose of a commercial red clover dietary supplement, we focused on the absorption of three such IF, irilone (IRI), prunetin (PRUN) and Pseudobaptigenin (PBAP). The compounds were measured as aglycones after enzymatic hydrolysis. A single intake of an amount of as low as 3.8 mg IRI (out of 38.8 mg IF in total) resulted in an IRI plasma concentration of 0.35 (sd 0.16) mum at 6.5 h post-ingestion. Compared to the plasma concentrations found for daidzein (0.39 mum) and genistein (0.06 mum), expected to be the main IF metabolites in plasma, the present findings indicate that IRI might possess a relatively high bioavailability. Furthermore, PRUN and PBAP were detected in human plasma for the first time.
Isoflavonoid composition of a callus culture of the relict tree Maackia amurensis Rupr. et Maxim.[Pubmed:18671403]
J Agric Food Chem. 2008 Aug 27;56(16):7023-31.
Isoflavonoids, an interesting and restricted group of secondary metabolites of legumes, exhibit estrogenic, antiangiogenic, and anticancer activities and are now popular as dietary supplements. Plant cell cultures that possess an increased ability to synthesize these metabolites were examined. During the investigation, cell cultures of the Far Eastern relict tree Maackia amurensis (Leguminosae) were established. A selection of seed-derived cell aggregates yielded the callus line designated A-18. This culture produces 20 isoflavonoids, namely, the isoflavones genistein, daidzein, formononetin, calycosin, derrone, and Pseudobaptigenin and their glycosylated conjugates genistin, 6''-O-malonylgenistin, ononin, 6''-O-malonylononin, daidzin, 3'-methoxydaidzin, 4'-O-beta-D-glucopyranosyldaidzin, 4'-O-beta-D-glucopyranosylgenistin, and 7-O-beta-D-glucopyranosylcalycosin; the pterocarpans maackiain and medicarpin and their glycosylated conjugates 6'-O-malonyl-3-O-beta-D-glucopyranosylmaackiain and 6'-O-malonyl-3-O-beta-D-glucopyranosylmedicarpin; and the new pterocarpan glucoside 6'-O-malonyl-3-O-beta-D-glucopyranosyl-6,6a-dehydromaackiain. These isoflavonoids, possessing a hepatoprotective activity, were stably produced by the A-18 calli for prolonged periods of observation.


