SYM 2206CAS# 173952-44-8 |
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 173952-44-8 | SDF | Download SDF |
| PubChem ID | 5039877 | Appearance | Powder |
| Formula | C20H22N4O3 | M.Wt | 366.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (272.92 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
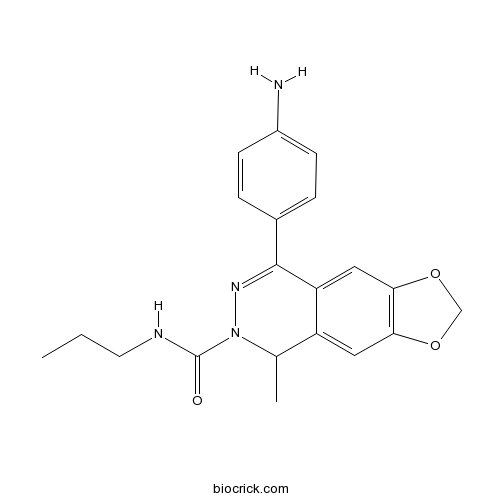
| Chemical Name | 8-(4-aminophenyl)-5-methyl-N-propyl-5H-[1,3]dioxolo[4,5-g]phthalazine-6-carboxamide | ||
| SMILES | CCCNC(=O)N1C(C2=CC3=C(C=C2C(=N1)C4=CC=C(C=C4)N)OCO3)C | ||
| Standard InChIKey | OFUDZKKOKPGXOH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22N4O3/c1-3-8-22-20(25)24-12(2)15-9-17-18(27-11-26-17)10-16(15)19(23-24)13-4-6-14(21)7-5-13/h4-7,9-10,12H,3,8,11,21H2,1-2H3,(H,22,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel, potent, non-competitive AMPA receptor antagonist (IC50 = 2.8 μM ). Acts allosterically at the same regulatory site as GYKI 52466 and 53655 and other benzodiazepines but does not bind to the central diazepine binding site. Selective for AMPA relative to kainate receptor sub-types. Similar potency to GYKI 53655. Anticonvulsant in vivo. |

SYM 2206 Dilution Calculator

SYM 2206 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7291 mL | 13.6455 mL | 27.2911 mL | 54.5822 mL | 68.2277 mL |
| 5 mM | 0.5458 mL | 2.7291 mL | 5.4582 mL | 10.9164 mL | 13.6455 mL |
| 10 mM | 0.2729 mL | 1.3646 mL | 2.7291 mL | 5.4582 mL | 6.8228 mL |
| 50 mM | 0.0546 mL | 0.2729 mL | 0.5458 mL | 1.0916 mL | 1.3646 mL |
| 100 mM | 0.0273 mL | 0.1365 mL | 0.2729 mL | 0.5458 mL | 0.6823 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SYM 2206 is a novel, potent, non-competitive AMPA receptor antagonist (IC50= 2.8 μM). IC50 value: 2.8 μM Target: AMPA receptor SYM 2206 exerts anticonvulsant effects, elevates the threshold for maximal electroshock-induced seizures in mice. TID20 and TID50(threshold increasing doses by 20% and 50%) values for SYM 2206 are 4.25 and 10.56 mg/kg in the MEST test in mice, respectively.
References:
[1]. Jarogniew J. Luszczki,et al. SYM 2206 (a potent non-competitive AMPA receptor antagonist) elevates the threshold for maximal electroshock-induced seizures in mice. Curr. Issues Pharm. Med. Sci., Vol. 27, No. 2, Pages 80-83
- Isogambogenin
Catalog No.:BCN3066
CAS No.:173938-23-3
- Atrasentan
Catalog No.:BCC1379
CAS No.:173937-91-2
- Gambogenic acid
Catalog No.:BCN3077
CAS No.:173932-75-7
- Corynoxine B
Catalog No.:BCN8454
CAS No.:17391-18-3
- Isocarapanaubine
Catalog No.:BCN1117
CAS No.:17391-09-2
- FR 171113
Catalog No.:BCC7734
CAS No.:173904-50-2
- Y-39983 dihydrochloride
Catalog No.:BCC4186
CAS No.:173897-44-4
- Swertiamarin
Catalog No.:BCN1116
CAS No.:17388-39-5
- H-Leu-OBzl.TosOH
Catalog No.:BCC2970
CAS No.:1738-77-8
- H-Gly-OBzl.TosOH
Catalog No.:BCC2948
CAS No.:1738-76-7
- H-Ser-OBzl.HCl
Catalog No.:BCC3030
CAS No.:1738-72-3
- H-Gly-OBzl.HCl
Catalog No.:BCC2949
CAS No.:1738-68-7
- Tanshinone IIB
Catalog No.:BCN1118
CAS No.:17397-93-2
- 22-Hydroxy-3-oxo-12-ursen-30-oic acid
Catalog No.:BCN1526
CAS No.:173991-81-6
- 5-Acetyl-6-hydroxy-2-(1-hydroxy-1-methylethyl)benzofuran
Catalog No.:BCN7495
CAS No.:173992-05-7
- Nepicastat
Catalog No.:BCC1795
CAS No.:173997-05-2
- Dehydroabietic acid
Catalog No.:BCN1119
CAS No.:1740-19-8
- Bevirimat
Catalog No.:BCC5312
CAS No.:174022-42-5
- Tomatine
Catalog No.:BCN2966
CAS No.:17406-45-0
- 3-(4-Pyridyl)-D-Alanine.2HCl
Catalog No.:BCC2650
CAS No.:174096-41-4
- Aristolochic acid D
Catalog No.:BCN2902
CAS No.:17413-38-6
- 3-Chloro-4-hydroxypiperidin-2-one
Catalog No.:BCN3992
CAS No.:174204-83-2
- Methyl 3-(2,4-dihydroxyphenyl)propionate
Catalog No.:BCN1525
CAS No.:17422-90-1
- Pancixanthone A
Catalog No.:BCN7379
CAS No.:174232-30-5
Kainate Receptors Play a Role in Modulating Synaptic Transmission in the Olfactory Bulb.[Pubmed:30213766]
Neuroscience. 2018 Nov 1;391:25-49.
Glutamate is the neurotransmitter used at most excitatory synapses in the mammalian brain, including those in the olfactory bulb (OB). There, ionotropic glutamate receptors including N-methyl-d-aspartate receptors (NMDARs) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) play a role in processes such as reciprocal inhibition and glomerular synchronization. Kainate receptors (KARs) represent another type of ionotropic glutamate receptor, which are composed of five (GluK1-GluK5) subunits. Whereas KARs appear to be heterogeneously expressed in the OB, evidence as to whether these KARs are functional, found at synapses, or modify synaptic transmission is limited. In the present study, coapplication of KAR agonists (kainate, SYM 2081) and AMPAR antagonists (GYKI 52466, SYM 2206) demonstrated that functional KARs are expressed by OB neurons, with a subset of receptors located at synapses. Application of kainate and the GluK1-selective agonist ATPA had modulatory effects on excitatory postsynaptic currents (EPSCs) evoked by stimulation of the olfactory nerve layer. Application of kainate and ATPA also had modulatory effects on reciprocal inhibitory postsynaptic currents (IPSCs) evoked using a protocol that evokes dendrodendritic inhibition. The latter finding suggests that KARs, with relatively slow kinetics, may play a role in circuits in which the relatively brief duration of AMPAR-mediated currents limits the role of AMPARs in synaptic transmission (e.g., reciprocal inhibition at dendrodendritic synapses). Collectively, our findings suggest that KARs, including those containing the GluK1 subunit, modulate excitatory and inhibitory transmission in the OB. These data further suggest that KARs participate in the regulation of synaptic circuits that encode odor information.
Kainic acid triggers oligodendrocyte precursor cell proliferation and neuronal differentiation from striatal neural stem cells.[Pubmed:17342781]
J Neurosci Res. 2007 May 1;85(6):1170-82.
Glutamate is an excitatory amino acid that serves important functions in mammalian brain development through alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)/ kainate receptor stimulation. Neural stem cells with self-renewal and multilineage potential are a useful tool to study the signals involved in the regulation of brain development. We have investigated the role played by AMPA/kainate receptors during the differentiation of neural stem cells derived from fetal rat striatum. The application of 1 and 10 microM kainic acid increased significantly the phosphorylation of the cyclic AMP response element binding protein (CREB), raised bromodeoxyuridine incorporation in O4-positive oligodendrocyte precursors, and increased the number of O1-positive cells in the cultures. Increased CREB phosphorylation and proliferation were prevented by the AMPA receptor antagonist 4-4(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphtha lazine (SYM 2206) and by protein kinase A and protein kinase C inhibitors. Cultures treated with 100 microM kainic acid showed decreased proliferation, a lower proportion of O1-positive cells, and apoptosis of O4-positive cells. None of these effects were prevented by SYM 2206, suggesting that kainate receptors take part in these events. We conclude that AMPA receptor stimulation by kainic acid promotes the proliferation of oligodendrocyte precursors derived from neural stem cells through a mechanism that requires the activation of CREB by protein kinase A and C. In the neurons derived from these cells, either AMPA or kainate receptor stimulation produces neuritic growth and larger cell bodies.
Use of [3H]fluorowillardiine to study properties of AMPA receptor allosteric modulators.[Pubmed:16256076]
Brain Res. 2006 Mar 3;1076(1):25-41.
Compounds which modulate AMPA receptor function through allosteric mechanisms were examined for their effect on the binding of the agonist [3H]fluorowillardiine (FW). Benzamide-type positive modulators (ampakinestrade mark) under all experimental circumstances increased [3H]FW binding to native receptors in rat brain membranes. Benzothiadiazide drugs had more variable effects ranging from large reductions with cyclothiazide and JM-13 to increases produced by more recent compounds like PEPA, D1 and LY392098. These effects on binding were moderately influenced by the assay conditions, including temperature and the presence or absence of thiocyanate. Significant changes in agonist binding were also produced by other modulatory agents such as noncompetitive blockers (GYKI 53655, SYM 2206), polycationic compounds (spermine, Naspm, philanthotoxin) and polyanionic compounds (Evans Blue, suramin, PPNDS). EC50 values usually were similar to those from physiological studies, which validates using binding tests to assess drug potencies. Moreover, direction and magnitude of the binding change (Emax) provide information about which kinetic aspects are affected by a drug. For example, the magnitude of the binding increase produced by positive modulators was strongly correlated with their ability to slow response deactivation in excised patch recordings. Binding also provides a reliable method to examine whether interactions between agents are competitive. Thus, thiocyanate did not significantly influence the EC50 of cyclothiazide, suggesting distinct sites of action. Taken together, [3H]FW binding can yield important information about drug-receptor and drug-drug interactions for a wide range of modulatory agents. One potential limitation of [3H]FW is a large preference for subunits GluR1 and GluR2 (KD 4-10 nM) over GluR3 and GluR4 (160-600 nM) which implies that tests with brain membranes preferentially reveal drug effects produced at the former two subunits. Lastly, data are shown which highlight the importance of optimizing experimental conditions in filtration assays, for instance by always including thiocyanate in wash buffers.
Glutamate-induced cobalt uptake elicited by kainate receptors in rat taste bud cells.[Pubmed:15703333]
Chem Senses. 2005 Feb;30(2):137-43.
Glutamate-induced cobalt uptake reveals non-N-methyl-D-aspartate (non-NMDA) glutamate receptors (GluRs) in rat taste bud cells. However, it is not known which type of non-NMDA glutamate receptors is involved. We used a cobalt staining technique combined with pharmacological tests for kainate or alpha-amino-3-hydroxy-5-methyl-isoxazole-propionic acid (AMPA) receptors and/or immunohistochemistry against subunits of GluRs to examine the presence of non-NMDA receptors in rat foliate tastebud cells. Cobalt uptake into taste cells was elicited by treating taste buds with glutamate, kainate or SYM 2081, a kainate receptor agonist. Treating taste buds with AMPA or fluorowillardiine did not stimulate significant cobalt uptake. Moreover, 6-cyano-7-nitro-quinoxaline-2, 3-dione significantly reduced cobalt staining elicited by glutamate or kainate receptor agonists, but SYM 2206, an AMPA receptor antagonist, did not. Immunohistochemistry against subunits of GluRs reveals GluR6 and KA1-like immunoreactivity. Moreover, most glutamate-induced cobalt-stained cells showed GluR6 and KA1-like immunoreactivity. These results suggest that glutamate-induced cobalt uptake in taste cells occurs mainly via kainate type GluRs.
Estradiol stimulation of pulsatile gonadotropin-releasing hormone secretion in vitro: correlation with perinatal exposure to sex steroids and induction of sexual precocity in vivo.[Pubmed:14988382]
Endocrinology. 2004 Jun;145(6):2775-83.
Our aim was to study the effect of estradiol (E2) on pulsatile GnRH secretion in vitro in relation to sex and development. When hypothalamic explants obtained from 5- and 15-d-old female rats were exposed to E2 (10(-7) m), a reduction of GnRH interpulse interval (IPI) occurred but not at 25 and 50 d of age. This effect was prevented by the estrogen receptor antagonist ICI 182.780 and the AMPA/kainate receptor antagonist DNQX but not by the AMPA and N-methyl-d-aspartate receptor antagonists SYM 2206 and MK-801. E2 did not affect GnRH IPI in hypothalamic explants obtained from male rats. Therefore, the possible relation between the female-specific effects of E2 in vitro and perinatal sexual differentiation was investigated. When using explants obtained from female rats masculinized through testosterone injection on postnatal d 1, E2 was no longer effective in vitro at 5 and 15 d. In addition, with explants obtained from male rats demasculinized through perinatal aromatase inhibitor treatment, E2 became capable of decreasing GnRH IPI in vitro at 15 d. To study the possible pathophysiological significance of early hypothalamic E2 effects, female rats received a single E2 injection on postnatal d 10. This resulted in reduced GnRH IPI in vitro on d 15 as well as advancement in age at vaginal opening and first estrus. In conclusion, E2 decreases the GnRH IPI in the immature female hypothalamus in vitro through a mechanism that depends on perinatal brain sexual differentiation and that could be involved in some forms of female precocious puberty.
Kindling enhances kainate receptor-mediated depression of GABAergic inhibition in rat granule cells.[Pubmed:12372022]
Eur J Neurosci. 2002 Sep;16(5):861-7.
Several lines of evidence indicate a substantial contribution of kainate receptors to temporal lobe seizures. The activation of kainate receptors located on hippocampal inhibitory interneurons was shown to reduce GABA release. A reduced GABA release secondary to kainate receptor activation could contribute to an enhanced seizure susceptibility. As the dentate gyrus serves a pivotal gating function in the spread of limbic seizures, we tested the role of kainate receptors in the regulation of GABA release in the dentate gyrus of control and kindled animals. Application of glutamate (100 micro m) in the presence of the NMDA receptor antagonist d-APV and the AMPA receptor antagonist, SYM 2206 caused a slight depression of evoked monosynaptic inhibitory postsynaptic currents (IPSCs) in control, but a substantial decrease in kindled dentate granule cells. The observation that kainate receptor activation altered paired-pulse depression and reduced the frequency of TTX-insensitive miniature IPSCs without affecting their amplitude is consistent with a presynaptic action on the inhibitory terminal to reduce GABA release. In kindled preparations, neither glutamate (100 micro m) nor kainate (10 micro m) applied in a concentration known to depolarize hippocampal interneurons led to an increase of the TTX-sensitive spontaneous IPSC frequency nor to changes of the postsynaptic membrane properties. Consistently, the inhibitory effect on evoked IPSCs was not affected by the presence of the GABAB receptor antagonist, CGP55845A, thus excluding a depression by an enhanced release of GABA acting on presynaptic GABAB receptors. The enhanced inhibition of GABA release following presynaptic kainate receptor activation favours a use-dependent hyperexcitability in the epileptic dentate gyrus.
Kindling induces transient NMDA receptor-mediated facilitation of high-frequency input in the rat dentate gyrus.[Pubmed:11353034]
J Neurophysiol. 2001 May;85(5):2195-202.
To elucidate the gating mechanism of the epileptic dentate gyrus on seizure-like input, we investigated dentate gyrus field potentials and granule cell excitatory postsynaptic potentials (EPSPs) following high-frequency stimulation (10-100 Hz) of the lateral perforant path in an experimental model of temporal lobe epilepsy (i.e., kindled rats). Although control slices showed steady EPSP depression at frequencies greater than 20 Hz, slices taken from animals 48 h after the last seizure presented pronounced EPSP facilitation at 50 and 100 Hz, followed by steady depression. However, 28 days after kindling, the EPSP facilitation was no longer detectable. Using the specific N-methyl-D-aspartate (NMDA) and RS-alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproponic acid (AMPA) receptor antagonists 2-amino-5-phosphonovaleric acid and SYM 2206, we examined the time course of alterations in glutamate receptor-dependent synaptic currents that parallel transient EPSP facilitation. Forty-eight hours after kindling, the fractional AMPA and NMDA receptor-mediated excitatory postsynaptic current (EPSC) components shifted dramatically in favor of the NMDA receptor-mediated response. Four weeks after kindling, however, AMPA and NMDA receptor-mediated EPSCs reverted to control-like values. Although the granule cells of the dentate gyrus contain mRNA-encoding kainate receptors, neither single nor repetitive perforant path stimuli evoked kainate receptor-mediated EPSCs in control or in kindled rats. The enhanced excitability of the kindled dentate gyrus 48 h after the last seizure, as well as the breakdown of its gating function, appear to result from transiently enhanced NMDA receptor activation that provides significantly slower EPSC kinetics than those observed in control slices and in slices from kindled animals with a 28-day seizure-free interval. Therefore, NMDA receptors seem to play a critical role in the acute throughput of seizure activity and in the induction of the kindled state but not in the persistence of enhanced seizure susceptibility.
Glutamate receptor activation in the kindled dentate gyrus.[Pubmed:10999529]
Epilepsia. 2000;41 Suppl 6:S100-3.
PURPOSE: The contribution of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate receptor activation to the enhanced seizure susceptibility of the dentate gyrus was investigated in an experimental model of temporal lobe epilepsy. METHODS: Using the specific NMDA and AMPA receptor antagonists D-APV and SYM 2206, we examined alterations in glutamate receptor-dependent synaptic currents 48 hours and 28 days after kindling in field-potential and voltage-clamp recordings. RESULTS: Forty-eight hours after kindling, the fractions of AMPA and NMDA receptor-mediated excitatory postsynaptic current components shifted dramatically in favor of the NMDA receptor-mediated response. Four weeks after kindling, however, AMPA and NMDA receptor-mediated excitatory postsynaptic currents reverted to control-like values. Neither single nor repetitive perforant path stimuli evoked kainate receptor-mediated excitatory postsynaptic currents in dentate gyrus granule cells of control or kindled rats. CONCLUSION: The enhanced excitability of the kindled dentate gyrus 48 hours after the last seizure most likely results from transiently enhanced NMDA receptor activation. The NMDA receptor seems to play a critical role in the induction of the kindled state rather than in the persistence of the enhanced seizure susceptibility.
Allosteric modulators of the AMPA receptor: novel 6-substituted dihydrophthalazines.[Pubmed:10098658]
Bioorg Med Chem Lett. 1999 Feb 22;9(4):539-42.
Novel analogs of the allosteric AMPA receptor modulator SYM 2206 have been prepared. Structure/activity correlations of these novel analogs and other dihydrophthalazines (DHPs) reveal the important contribution of the heteroatom-based aryl substituents in this class of noncompetitive inhibitors. One of the analogs (6, SYM 2189) is equipotent with the early series, but with reduced sedation.
Kainate receptor agonists, antagonists and allosteric modulators.[Pubmed:11945137]
Curr Pharm Des. 2002;8(10):873-85.
Interest in kainate receptors has increased over the past few years. Our understanding of their physiology and pharmacology has improved markedly since their original cloning and expression in the early 1990s. For example, agonist profiles at recombinant kainate receptors have been used to identify and distinguish kainate receptors in neurons. Furthermore, the development of selective antagonists for kainate receptor subtypes has increased our understanding of the functional roles of kainate receptors in neurons and synaptic transmission. In this review we described the activity of agonists and antagonists at kainate receptors and their selectivity profiles at NMDA and non-NMDA receptors.
Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord.[Pubmed:9923678]
Nature. 1999 Jan 14;397(6715):161-4.
Glutamate, the major excitatory neurotransmitter in the central nervous system, activates three different receptors that directly gate ion channels, namely receptors for AMPA (alpha-amino-3-hydroxy-5-methyl isoxozole propionic acid), NMDA (N-methyl-D-aspartate), and kainate, a structural analogue of glutamate. The contribution of AMPA and NMDA receptors to synaptic transmission and plasticity is well established. Recent work on the physiological function of kainate receptors has focused on the hippocampus, where repetitive activation of the mossy-fibre pathway generates a slow, kainate-receptor-mediated excitatory postsynaptic current (EPSC). Here we show that high-intensity single-shock stimulation (of duration 200 microseconds) of primary afferent sensory fibres produces a fast, kainate-receptor-mediated EPSC in the superficial dorsal horn of the spinal cord. Activation of low-threshold afferent fibres generates typical AMPA-receptor-mediated EPSCs only, indicating that kainate receptors may be restricted to synapses formed by high-threshold nociceptive (pain-sensing) and thermoreceptive primary afferent fibres. Consistent with this possibility, kainate-receptor-mediated EPSCs are blocked by the analgesic mu-opiate-receptor agonist Damgo and spinal blockade of both kainate and AMPA receptors produces antinociception. Thus, spinal kainate receptors contribute to transmission of somatosensory inputs from the periphery to the brain.


