SachaconitineCAS# 1361-02-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1361-02-0 | SDF | Download SDF |
| PubChem ID | 101297639 | Appearance | Powder |
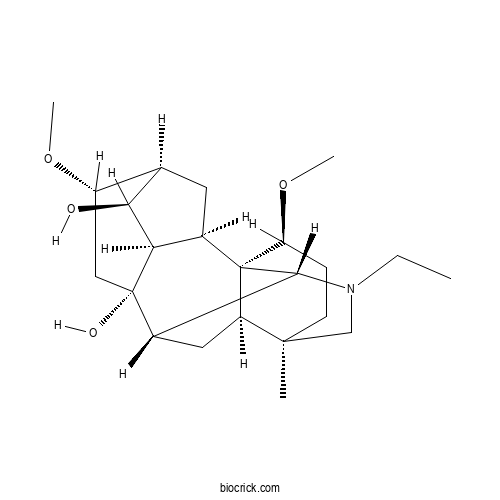
| Formula | C23H37NO4 | M.Wt | 391.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Vilmorrianine D | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,2R,3R,4S,5S,6S,8S,9S,10R,13R,16S,17R)-11-ethyl-6,16-dimethoxy-13-methyl-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecane-4,8-diol | ||
| SMILES | CCN1CC2(CCC(C34C2CC(C31)C5(CC(C6CC4C5C6O)OC)O)OC)C | ||
| Standard InChIKey | NGWMZXLZSGJSRI-VHNKBEDGSA-N | ||
| Standard InChI | InChI=1S/C23H37NO4/c1-5-24-11-21(2)7-6-17(28-4)23-13-8-12-15(27-3)10-22(26,18(13)19(12)25)14(20(23)24)9-16(21)23/h12-20,25-26H,5-11H2,1-4H3/t12-,13-,14+,15+,16-,17+,18-,19+,20-,21+,22+,23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sachaconitine Dilution Calculator

Sachaconitine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5543 mL | 12.7714 mL | 25.5428 mL | 51.0856 mL | 63.857 mL |
| 5 mM | 0.5109 mL | 2.5543 mL | 5.1086 mL | 10.2171 mL | 12.7714 mL |
| 10 mM | 0.2554 mL | 1.2771 mL | 2.5543 mL | 5.1086 mL | 6.3857 mL |
| 50 mM | 0.0511 mL | 0.2554 mL | 0.5109 mL | 1.0217 mL | 1.2771 mL |
| 100 mM | 0.0255 mL | 0.1277 mL | 0.2554 mL | 0.5109 mL | 0.6386 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14-O-Acetylsachaconitine
Catalog No.:BCN0559
CAS No.:102719-98-2
- Falconeridine
Catalog No.:BCN0558
CAS No.:121880-22-6
- Haplamide
Catalog No.:BCN0557
CAS No.:31991-78-3
- Velutinam
Catalog No.:BCN0556
CAS No.:146428-62-8
- (19S,23E)-5β,19-Epoxy-19-methoxycucurbita-6,23,25-trien-3β-ol
Catalog No.:BCN0555
CAS No.:874287-87-3
- Glochidiolide
Catalog No.:BCN0554
CAS No.:213528-23-5
- 2''-O-Rhamnosylswertisin
Catalog No.:BCN0553
CAS No.:83889-78-5
- Squamolone
Catalog No.:BCN0552
CAS No.:40451-67-0
- 2-Hydroxybenzylcyanide
Catalog No.:BCN0551
CAS No.:14714-50-2
- Methyl 2-(2,3-dihydroxyphenyl)acetate
Catalog No.:BCN0550
CAS No.:168481-87-6
- Chrysin 7-O-neohesperidoside
Catalog No.:BCN0549
CAS No.:35775-46-3
- Porphyroxine
Catalog No.:BCN0548
CAS No.:18104-24-0
- Philippin A
Catalog No.:BCN0561
CAS No.:1848254-99-8
- 2,3-Dihydroxybenzeneacetonitrile
Catalog No.:BCN0562
CAS No.:1513469-96-9
- Lespedezaflavanone H
Catalog No.:BCN0563
CAS No.:138822-59-0
- Glaucocalyxin A diacetate
Catalog No.:BCN0564
CAS No.:79498-37-6
- (19S,23R)-5β,19-Epoxy-19,23-dimethoxycucurbita-6,24-dien-3β-ol
Catalog No.:BCN0565
CAS No.:1474062-47-9
- 8-(1,1-Dimethylallyl)genistein
Catalog No.:BCN0566
CAS No.:651750-08-2
- 5-Hydroxyoxindole
Catalog No.:BCN0567
CAS No.:3416-18-0
- 7,4'-Di-O-methylvitexin 2''-O-rhamnoside
Catalog No.:BCN0568
CAS No.:1236226-98-4
- (19R,23S)-5β,19-Epoxy-19,23-dimethoxycucurbita-6,24-dien-3β-ol
Catalog No.:BCN0569
CAS No.:2281872-79-3
- 3β-(4-Hydroxybenzoyloxy)-21β,29-dihydroxyserrat-14-en-24-oic acid
Catalog No.:BCN0570
CAS No.:1217268-13-7
- 3β-Vanilloyloxy-21β,29-dihydroxyserrat-14-en-24-oic acid
Catalog No.:BCN0571
CAS No.:1217268-14-8
- 16α-Hydroxy-19,20-epoxy-20β-methoxy-ent-kaurane
Catalog No.:BCN0572
CAS No.:197369-57-6
[Diterpenoid alkaloids from roots of Aconitum kongboense].[Pubmed:34581046]
Zhongguo Zhong Yao Za Zhi. 2021 Sep;46(17):4424-4432.
The chemical constituents from the roots of Aconitum kongboense were studied. Twenty-five diterpenoid alkaloids were isolated from the 95% methanol extract of the roots of A. kongboense by silica gel, reverse-phase silica gel and basic alumina column chromatography. They included a new aconitine-type diterpenoid alkaloid, named as kongboensenine(1), and twenty-four known ones(2-25), i.e., acotarine F(2), acotarine G(3), 14-acetyltalatisamine(4), talatisamine(5), indaconitine(6), yunaconitine(7), chasmanine(8), 6-epi-foresticine(9), homochasmanine(10), 8-deacetyl-yunaconitine(11), chasmaconitine(12), ajaconine(13), franchetine(14), ezochasmanine(15), crassicautine(16), 14-O-deacylcrassicausine(17), genicunine A(18), falconeridine(19), Sachaconitine(20), liljestrandisine(21), 8-methyl-14-acetyltalatisamine(22), kongboendine(23), 14-benzoylchasmanine(24) and pseudaconine(25). Their structures were elucidated by common spectroscopic methods including high-resolution electrospray ionization mass spectrometry(HR-ESI-MS) and nuclear magnetic resonance(NMR) techniques. Compounds 2-4, 10, 13, 15-19 and 21-22 were isolated from this plant for the first time. Experimental results showed that all compounds did not have a significant inhibitory activity against acetylcholinesterase(AChE).
Feeding deterrents from Aconitum episcopale roots against the red flour beetle, Tribolium castaneum.[Pubmed:21417277]
J Agric Food Chem. 2011 Apr 27;59(8):3701-6.
The screening for insecticidal principles from several Chinese medicinal herbs showed that the ethanol extract of Aconitum episcopale roots possessed significant feeding deterrence against the red flour beetle, Tribolium castaneum . From the ethanol extract, six feeding deterrents were isolated by bioassay-guided fractionation. The compounds were identified as chasmanine, crassicauline A, karacoline, Sachaconitine, talatisamine, and yunaconitine from their spectroscopic data. Chasmanine, talatisamine, karacoline, and Sachaconitine exhibited feeding deterrent activity against T. castaneum adults, with EC(50) values of 297.0, 342.8, 395.3, and 427.8 ppm, respectively. Yunaconitine and crassicauline A also possessed feeding deterrent activity against T. castaneum adults, with EC(50) values of 653.4 and 1134.5 ppm, respectively.
[Diterpenoid alkaloids from roots of Aconitum recemulosum and their inhibitory effects on PAF-induced platelet aggregation].[Pubmed:19894538]
Zhongguo Zhong Yao Za Zhi. 2009 Aug;34(15):1935-7.
OBJECTIVE: To study diterpenoid alkaloids from the roots of Aconitum recemulosum, and their inhibitory effects on PAF-induced platelet aggregation. METHOD: The root of A. recemulosum was extracted with 95% EtOH. The total alkaloids extracted were isolated and purified by several kinds of column chromatography over silica gel, RP-18, and Sephadex LH-20, and identified based on spectral analysis. And the inhibitory effects of isolated compounds on PAF-induced platelet aggregation were detected. RESULT: Five alkaloids were isolated and identified as Sachaconitine (1), 14-acetylSachaconitine (2), hemsleyanine C (3), circinasine A (4), and talatisamine (5). The results showed compounds 1 and 2 have moderate inhibition effect on PAF. CONCLUSION: Compounds 1-5 were firstly isolated from this plant. Furthermore, compounds 1 and 2 possessed moderate inhibitory effects on PAF-induced platelet aggregation.
C19-diterpenoid alkaloids from Aconitum hemsleyanum var. circinatum.[Pubmed:17432903]
J Nat Prod. 2007 May;70(5):876-9.
Seven new C19-diterpenoid alkaloids, circinasines A-G (1-7), together with six known compounds, talatisamine, yunaconitine, senbusine A, Sachaconitine, hemsleyanisine, and isohemsleyanisine, were isolated from the roots of Aconitum hemsleyanum var. circinatum. The structures of 1-7 were determined by the interpretation of spectroscopic data and by the single-crystal X-ray crystallographic analysis of 6 and the acetonide derivative of 1. In addition, the structures of hemsleyanisine and isohemsleyanisine were revised from 8 and 9 to 10 and 11, respectively.
Norditerpene and diterpene alkaloids from Aconitum variegatum.[Pubmed:15797610]
Phytochemistry. 2005 Apr;66(7):837-46.
Aerial parts of Aconitum variegatum L. from the Pyrenees furnished four norditerpene alkaloids, 16 beta-hydroxycardiopetaline, 8-ethoxySachaconitine, 14-acetylgenicunine B, N-deethyl-N-19-didehydroSachaconitine, five diterpene alkaloids 15-veratroyldictizine, 15-veratroyl-17-acetyldictizine, 15-veratroyl-17-acetyl-19-oxodictizine, N-ethyl-1 alpha-hydroxy-17-veratroyldictizine, variegatine and the known alkaloids Sachaconitine, 14-O-acetylSachaconitine, karakoline, talatizamine, 10-hydroxytalatizamine, 14-acetyltalatizamine, 14-acetyl-10-hydroxytalatizamine, N-methylarmepavine, pengshenin B, delsoline, dihydrodelsoline, delcosine and genicunin B. Structures of the alkaloids were established by MS, 1D- and 2D-NMR techniques.


