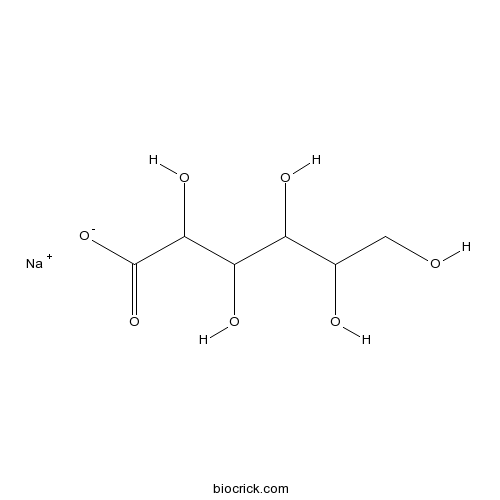
Sodium GluconateCAS# 527-07-1 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 527-07-1 | SDF | Download SDF |
| PubChem ID | 517056 | Appearance | Powder |
| Formula | C6H11NaO7 | M.Wt | 218.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | D-Gluconic acid sodium salt; Sodium D-gluconate; D-Gluconate sodium salt | ||
| Solubility | H2O : ≥ 100 mg/mL (458.42 mM) DMSO : 1 mg/mL (4.58 mM; ultrasonic and warming and heat to 60°C) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | sodium;2,3,4,5,6-pentahydroxyhexanoate | ||
| SMILES | C(C(C(C(C(C(=O)[O-])O)O)O)O)O.[Na+] | ||
| Standard InChIKey | UPMFZISCCZSDND-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C6H12O7.Na/c7-1-2(8)3(9)4(10)5(11)6(12)13;/h2-5,7-11H,1H2,(H,12,13);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sodium gluconate is a corrosion and scale inhibitor of ordinary steel in simulated cooling water. References: | |||||

Sodium Gluconate Dilution Calculator

Sodium Gluconate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5842 mL | 22.9211 mL | 45.8421 mL | 91.6842 mL | 114.6053 mL |
| 5 mM | 0.9168 mL | 4.5842 mL | 9.1684 mL | 18.3368 mL | 22.9211 mL |
| 10 mM | 0.4584 mL | 2.2921 mL | 4.5842 mL | 9.1684 mL | 11.4605 mL |
| 50 mM | 0.0917 mL | 0.4584 mL | 0.9168 mL | 1.8337 mL | 2.2921 mL |
| 100 mM | 0.0458 mL | 0.2292 mL | 0.4584 mL | 0.9168 mL | 1.1461 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sodium gluconate is the sodium salt of gluconic acid widely used in food and pharmaceutical industry.
- D-Penylalaninol
Catalog No.:BCC2715
CAS No.:5267-64-1
- A23187, free acid
Catalog No.:BCC6980
CAS No.:52665-69-7
- Kirenol
Catalog No.:BCN5682
CAS No.:52659-56-0
- Anisodine
Catalog No.:BCN1868
CAS No.:52646-92-1
- Siegeskaurolic acid
Catalog No.:BCN6982
CAS No.:52645-97-3
- Dehydroadynerigenin digitaloside
Catalog No.:BCN4623
CAS No.:52628-62-3
- Huwentoxin IV
Catalog No.:BCC6270
CAS No.:526224-73-7
- Gnetofuran B
Catalog No.:BCN7764
CAS No.:526214-79-9
- Isomorellic acid
Catalog No.:BCN3074
CAS No.:5262-69-1
- Ponicidin
Catalog No.:BCN3231
CAS No.:52617-37-5
- Deacetylasperulosidic acid methyl ester
Catalog No.:BCN1427
CAS No.:52613-28-2
- Epipterosin L
Catalog No.:BCN5680
CAS No.:52611-75-3
- Azomycin
Catalog No.:BCC5315
CAS No.:527-73-1
- Herbacetin
Catalog No.:BCN1268
CAS No.:527-95-7
- Ginsenoside Rd
Catalog No.:BCN1074
CAS No.:52705-93-8
- Scillascillin
Catalog No.:BCN5684
CAS No.:52706-07-7
- Isoelemicin
Catalog No.:BCN4760
CAS No.:5273-85-8
- beta-Asarone
Catalog No.:BCN5685
CAS No.:5273-86-9
- Medicarpin 3-O-glucoside
Catalog No.:BCN7773
CAS No.:52766-70-8
- PHA 568487
Catalog No.:BCC7574
CAS No.:527680-57-5
- H-D-Phe(3,4-DiCl)-OH
Catalog No.:BCC3179
CAS No.:52794-98-6
- H-Phe(3,4-DiCl)-OH
Catalog No.:BCC3178
CAS No.:52794-99-7
- Magnolol
Catalog No.:BCN5687
CAS No.:528-43-8
- Fisetin
Catalog No.:BCN5024
CAS No.:528-48-3
A simple novel approach for real-time monitoring of sodium gluconate production by on-line physiological parameters in batch fermentation by Aspergillus niger.[Pubmed:26706727]
Bioresour Technol. 2016 Feb;202:133-41.
In this paper, approach for real-time monitoring of Sodium Gluconate (SG) fermentation was established for the first time by the equations which can calculate real-time key-parameters by on-line physiological data. Based on this approach, limiting factors were found out in initial fermentation F1 and then step-wise agitation increase and improved medium recipe were proposed in fermentation F2 and F3, respectively. The highest average SG production rate (16.58+/-0.91 g L(-1) h(-1)) was achieved in fermentation F3, which was 104.2% and 48.0% higher than those in fermentation F1 and F2, respectively. Meanwhile, due to shorter fermentation period (decreased from 34 h to 18.7 h), lower biomass (about 1.5 g L(-1)) and less by-product accumulation, the overall yield of 0.943+/-0.012 (mol mol(-1)) in fermentation F3 increased more than 16.0% compared to fermentation F1. This approach had been successfully applied to industrial fermentation and greatly improved SG production.
[Efficacy of sodium hydroxide at 2.5 %, chlorhexidine gluconate at 0.5 % and calcium hydroxide against Candida albicans].[Pubmed:27198757]
J Mycol Med. 2016 Dec;26(4):317-322.
INTRODUCTION: Endodontic flora is dominated in the apical part of the channels by strict anaerobic and some facultative anaerobic bacteria but also by Candida yeasts, especially Candida albicans species that are involved in the maintenance and persistence of endodontic infections. Their elimination of the canal system in practice by chemo-mechanical methods of disinfection is not always guaranteed. Thus, this in vitro study was performed to determine the sensitivity of C. albicans with sodium hypochlorite (NaOCl) dosed at 2.5 %, the chlorhexidine digluconate 0.5 % and calcium hydroxide used in inter-session medication. METHODS: The diffusion method was used initially to test the sensitivity of C. albicans strains with the above products. Then a dilution technique has allowed us to determine the minimum inhibitory concentration of these active products on C. albicans. RESULTS: Strains from infected pulp teeth of patients showed a sensitivity of C. albicans to sodium hypochlorite to a minimum inhibitory concentration less than 70mug/mL and 30mug/mL for chlorhexidine. CONCLUSION: This study demonstrated a sensitivity of C. albicans to sodium hypochlorite and chlorhexidine.
High efficiency cell-recycle continuous sodium gluconate production by Aspergillus niger using on-line physiological parameters association analysis to regulate feed rate rationally.[Pubmed:27611026]
Bioresour Technol. 2016 Nov;220:433-441.
In this paper, a system of cell-recycle continuous fermentation for Sodium Gluconate (SG) production by Aspergillus niger (A. niger) was established. Based on initial continuous fermentation result (100.0h) with constant feed rate, an automatic feedback strategy to regulate feed rate using on-line physiological parameters (OUR and DO) was proposed and applied successfully for the first time in the improved continuous fermentation (240.5h). Due to less auxiliary time, highest SG production rate (31.05+/-0.29gL(-1)h(-1)) and highest yield (0.984+/-0.067molmol(-1)), overall SG production capacity (975.8+/-5.8gh(-1)) in 50-L fermentor of improved continuous fermentation increased more than 300.0% compared to that of batch fermentation. Improvement of mass transfer and dispersed mycelia morphology were the two major reasons responsible for the high SG production rate. This system had been successfully applied to industrial fermentation and SG production was greatly improved.
Safety of Intravenous Iron in Hemodialysis: Longer-term Comparisons of Iron Sucrose Versus Sodium Ferric Gluconate Complex.[Pubmed:28063734]
Am J Kidney Dis. 2017 Jun;69(6):771-779.
BACKGROUND: Controversy exists about any differences in longer-term safety across different intravenous iron formulations routinely used in hemodialysis (HD) patients. We exploited a natural experiment to compare outcomes of patients initiating HD therapy in facilities that predominantly (in >/=90% of their patients) used iron sucrose versus sodium ferric gluconate complex. STUDY DESIGN: Retrospective cohort study of incident HD patients. SETTING & PARTICIPANTS: Using the US Renal Data System, we hard-matched on geographic region and center characteristics HD facilities predominantly using ferric gluconate with similar ones using iron sucrose. Subsequently, incident HD patients were assigned to their facility iron formulation exposure. INTERVENTION: Facility-level use of iron sucrose versus ferric gluconate. OUTCOMES: Patients were followed up for mortality from any, cardiovascular, or infectious causes. Medicare-insured patients were followed up for infectious and cardiovascular (stroke or myocardial infarction) hospitalizations and for composite outcomes with the corresponding cause-specific deaths. MEASUREMENTS: HRs. RESULTS: We matched 2,015 iron sucrose facilities with 2,015 ferric gluconate facilities, in which 51,603 patients (iron sucrose, 24,911; ferric gluconate, 26,692) subsequently initiated HD therapy. All recorded patient characteristics were balanced between groups. Over 49,989 person-years, 10,381 deaths (3,908 cardiovascular and 1,209 infectious) occurred. Adjusted all-cause (HR, 0.98; 95% CI, 0.93-1.03), cardiovascular (HR, 0.96; 95% CI, 0.89-1.03), and infectious mortality (HR, 0.98; 95% CI, 0.86-1.13) did not differ between iron sucrose and ferric gluconate facilities. Among Medicare beneficiaries, no differences between ferric gluconate and iron sucrose facilities were observed in fatal or nonfatal cardiovascular events (HR, 1.01; 95% CI, 0.93-1.09). The composite infectious end point occurred less frequently in iron sucrose versus ferric gluconate facilities (HR, 0.92; 95% CI, 0.88-0.96). LIMITATIONS: Unobserved selection bias from nonrandom treatment assignment. CONCLUSIONS: Patients initiating HD therapy in facilities almost exclusively using iron sucrose versus ferric gluconate had similar longer-term outcomes. However, there was a small decrease in infectious hospitalizations and deaths in patients dialyzing in facilities predominantly using iron sucrose. This difference may be due to residual confounding, random chance, or a causal effect.


