AzomycinCAS# 527-73-1 |
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 527-73-1 | SDF | Download SDF |
| PubChem ID | 10701 | Appearance | Powder |
| Formula | C3H3N3O2 | M.Wt | 113.07 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 160 mg/mL (1415.05 mM; Need ultrasonic) | ||
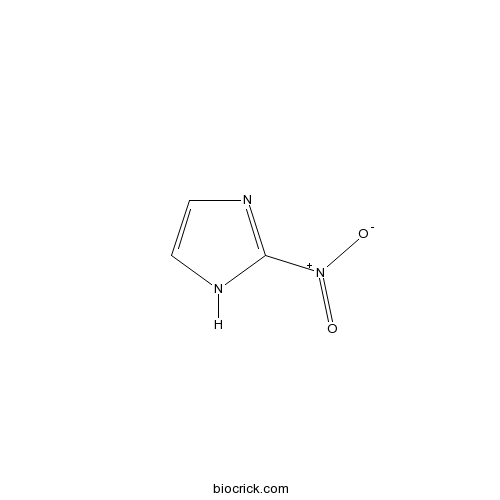
| Chemical Name | 2-nitro-1H-imidazole | ||
| SMILES | C1=CN=C(N1)[N+](=O)[O-] | ||
| Standard InChIKey | YZEUHQHUFTYLPH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C3H3N3O2/c7-6(8)3-4-1-2-5-3/h1-2H,(H,4,5) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Azomycin Dilution Calculator

Azomycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.8441 mL | 44.2204 mL | 88.4408 mL | 176.8816 mL | 221.102 mL |
| 5 mM | 1.7688 mL | 8.8441 mL | 17.6882 mL | 35.3763 mL | 44.2204 mL |
| 10 mM | 0.8844 mL | 4.422 mL | 8.8441 mL | 17.6882 mL | 22.1102 mL |
| 50 mM | 0.1769 mL | 0.8844 mL | 1.7688 mL | 3.5376 mL | 4.422 mL |
| 100 mM | 0.0884 mL | 0.4422 mL | 0.8844 mL | 1.7688 mL | 2.211 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sodium Gluconate
Catalog No.:BCC4721
CAS No.:527-07-1
- D-Penylalaninol
Catalog No.:BCC2715
CAS No.:5267-64-1
- A23187, free acid
Catalog No.:BCC6980
CAS No.:52665-69-7
- Kirenol
Catalog No.:BCN5682
CAS No.:52659-56-0
- Anisodine
Catalog No.:BCN1868
CAS No.:52646-92-1
- Siegeskaurolic acid
Catalog No.:BCN6982
CAS No.:52645-97-3
- Dehydroadynerigenin digitaloside
Catalog No.:BCN4623
CAS No.:52628-62-3
- Huwentoxin IV
Catalog No.:BCC6270
CAS No.:526224-73-7
- Gnetofuran B
Catalog No.:BCN7764
CAS No.:526214-79-9
- Isomorellic acid
Catalog No.:BCN3074
CAS No.:5262-69-1
- Ponicidin
Catalog No.:BCN3231
CAS No.:52617-37-5
- Deacetylasperulosidic acid methyl ester
Catalog No.:BCN1427
CAS No.:52613-28-2
- Herbacetin
Catalog No.:BCN1268
CAS No.:527-95-7
- Ginsenoside Rd
Catalog No.:BCN1074
CAS No.:52705-93-8
- Scillascillin
Catalog No.:BCN5684
CAS No.:52706-07-7
- Isoelemicin
Catalog No.:BCN4760
CAS No.:5273-85-8
- beta-Asarone
Catalog No.:BCN5685
CAS No.:5273-86-9
- Medicarpin 3-O-glucoside
Catalog No.:BCN7773
CAS No.:52766-70-8
- PHA 568487
Catalog No.:BCC7574
CAS No.:527680-57-5
- H-D-Phe(3,4-DiCl)-OH
Catalog No.:BCC3179
CAS No.:52794-98-6
- H-Phe(3,4-DiCl)-OH
Catalog No.:BCC3178
CAS No.:52794-99-7
- Magnolol
Catalog No.:BCN5687
CAS No.:528-43-8
- Fisetin
Catalog No.:BCN5024
CAS No.:528-48-3
- Delphinidin chloride
Catalog No.:BCN3015
CAS No.:528-53-0
Synthesis and Biological Evaluation of Iodoglucoazomycin (I-GAZ), an Azomycin-Glucose Adduct with Putative Applications in Diagnostic Imaging and Radiotherapy of Hypoxic Tumors.[Pubmed:27377671]
ChemMedChem. 2016 Aug 5;11(15):1638-45.
IodoglucoAzomycin (I-GAZ; N-(2-iodo-3-(6-O-glucosyl)propyl)-2-nitroimidazole), a non-glycosidic nitroimidazole-6-O-glucose adduct, was synthesized, radioiodinated, and evaluated as a substrate of glucose transporter 1 (GLUT1) for radiotheranostic (therapy+diagnostic) management of hypoxic tumors. Nucleophilic iodination of the nosylate synthon of I-GAZ followed by deprotection afforded I-GAZ in 74 % overall yield. I-GAZ was radioiodinated via 'exchange' labeling using [(123/131) I]iodide (50-70 % RCY) and then purified by Sep-Pak (>96 % RCP). [(131) I]I-GAZ was stable in 2 % ethanolic solution in sterile water for 14 days when stored at 5 degrees C. In cell culture, I-GAZ was found to be nontoxic to EMT-6 cells at concentrations <0.5 mm, and weakly radiosensitizing (SER 1.1 at 10 % survival of EMT-6 cells; 1.2 at 0.1 % survival in MCF-7 cells). The hypoxic/normoxic uptake ratio of [(123) I]I-GAZ in EMT-6 cells was 1.46 at 2 h, and under normoxic conditions the uptake of [(123) I]I-GAZ by EMT-6 cells was unaltered in the presence of 5 mm glucose. The biodistribution of [(131) I]I-GAZ in EMT-6 tumor-bearing Balb/c mice demonstrated rapid clearance from blood and extensive renal and hepatic excretion. Tumor/blood and tumor/muscle ratios reached approximately 3 and 8, respectively, at 4 h post-injection. Regression analysis of the first order polynomial plots of the blood and tumor radioactivity concentrations supported a perfusion-excretion model with low hypoxia-dependent binding. [(131) I]I-GAZ was found to be stable in vivo, and did not deiodinate.
beta -[18F]Fluoro Azomycin Arabinoside (beta -[18F]FAZA): Synthesis, Radiofluorination and Preliminary PET Imaging of Murine A431 Tumors.[Pubmed:28294075]
Curr Radiopharm. 2017;10(2):93-101.
BACKGROUND: 1-alpha-D-(5-Deoxy-5-[18F]fluoroarabinofuranosyl)-2-nitroimidazole([18F] FAZA) is a PET radiotracer that demonstrates excellent potential in imaging regional hypoxia, and is clinically used in diagnosing a wide range of solid tumors in cancer patients. [18F]FAZA, however, is radiofluorinated in only moderate recovered radiochemical yield (rRCY, ~12%). It is postulated that the relative stability of the C1' beta-anomeric bond at C5' will make 1-beta-D-(5-fluoro-5-deoxyarabinofuranosyl)-2-nitroimidazole (beta-FAZA), the beta-conformer of FAZA, an attractive candidate for clinical hypoxia imaging. OBJECTIVES: The principle goals were to synthesize beta-FAZA and beta-Ac2TsAZA, the radiofluorination precursor, to establish the radiofluorination chemistry leading to beta-[18F]FAZA, and to investigate the biodistribution of beta-[18F]FAZA in an animal tumor-bearing model using PET imaging. METHODS: The appropriately-protected furanose sugar was coupled with 2-nitroimidazole to afford 1-beta-D-(2,3-di-O-acetylarabinofuranosyl)-2-nitroimidazole (beta-Ac2AZA). Fluorination of beta-Ac2AZA with DAST, followed by alkaline hydrolysis, afforded beta-FAZA (21%). The radiolabeling synthon, 1-beta-D-(5-O-toluenesulfonyl-2,3-di-O-acetylarabinofuranosyl)-2-nitroimidazole (beta-Ac2TsAZA), on radiofluorination using the 18F/K222 complex under various reaction conditions, followed by base-catalyzed deacetylation, afforded beta-[18F]FAZA. beta-[18F]FAZA was radiochemically stable for at least 8 h when stored in aqueous ethanol (8%) at 22 degrees C. A preliminary PET imaging-based biodistribution study of beta-[18F]FAZA was performed in A431 tumor-bearing nude mice. RESULTS: beta-FAZA and beta-Ac2TsAZA were synthesized in satisfactory yield. Radiochemistry of [18F]FAZA was established. PET images showed strong uptake in hypoxic regions of the tumor. CONCLUSION: The synthesis of beta-FAZA and beta-[18F]FAZA are reported. Radiofluorination of beta-Ac2TsAZA and the deprotection of beta-Ac2[18F]FAZA were facile, but led to a more complex mixture of radiofluorinated by-products than observed with the corresponding precursor of alpha-[18F]FAZA. PET images were indicative of hypoxia-selective accumulation of beta-[18F]FAZA in tumor.
In vitro and in vivo evaluation of [(18)F]F-GAZ, a novel oxygen-mimetic azomycin-glucose conjugate, for imaging hypoxic tumor.[Pubmed:22746267]
Cancer Biother Radiopharm. 2012 Oct;27(8):473-80.
Several F-18-labeled 2-nitroimidazole (Azomycin) derivatives have been proposed for imaging hypoxia using positron emission tomography (PET). Their cell penetration is based on passive diffusion, which limits their intracellular concentration maxima. The purpose of this study was to investigate the uptake of N-(2-[(18)F]fluoro-3-(6-O-glucosyl)propyl-Azomycin ([(18)F]F-GAZ), a new Azomycin-glucose conjugate, in vitro and in vivo. [(18)F]F-GAZ was synthesized from its tetraacetyl nosylate precursor by nucleophilic radiofluorination. [(18)F]F-GAZ was evaluated in vivo in EMT-6 tumor-bearing Balb/C mice utilizing the PET and biodistribution analysis. In vitro uptake of [(18)F]FDG by EMT-6 cells was measured in the presence of unlabeled F-GAZ, 2-FDG, and D-glucose. [(18)F]F-GAZ was rapidly cleared from all tissues, including the blood pool and kidneys, with ultimate accumulation in the urinary bladder. Uptake of tracer doses of [(18)F]F-GAZ into EMT-6 tumors was fast, reaching a standardized uptake value of 0.66+/-0.05 within 5-6 minutes postinjection (p.i.), and decreased to 0.24+/-0.04 by 60 minutes p.i. (n=6). A tumor-muscle ratio of 1.87+/-0.18 was observed after 60 minutes. Total uptake of [(18)F]F-GAZ in tumors (60 minutes) amounted to 1.25%+/-0.15% ID/g versus 0.61%+/-0.14% ID/g (n=4) in muscle. Similar biodistribution and excretion were observed using carrier-added (100 mg/kg) doses of F-GAZ. In vitro, D-glucose and unlabeled 2-FDG were two orders of magnitude more potent than F-GAZ as competitive inhibitors of [(18)F]FDG uptake into EMT-6 cells. Besides its interaction with glucose transporters, F-GAZ seems to be not transported in the presence of glucose. Furthermore, [(18)F]F-GAZ is unlikely to be effective as a hypoxia imaging agent. The low in vivo toxicity and substantial retention in tumor observed at high doses of F-GAZ do provide rationale for further testing as a radiosensitizer for external beam radiation therapy of radioresistant, hypoxic tumors.
[(18)F]Fluoro-azomycin-2 -deoxy-beta-d-ribofuranoside - A new imaging agent for tumor hypoxia in comparison with [(18)F]FAZA.[Pubmed:27693670]
Nucl Med Biol. 2016 Dec;43(12):759-769.
INTRODUCTION: Radiolabeled 2-nitroimidazoles (Azomycins) are a prominent class of biomarkers for PET imaging of hypoxia. [(18)F]Fluoro-Azomycin-alpha-arabinoside ([(18)F]FAZA) - already in clinical use - may be seen as alpha-configuration nucleoside, but enters cells only via diffusion and is not transported by cellular nucleoside transporters. To enhance image contrast in comparison to [(18)F]FAZA our objective was to (18)F-radiolabel an Azomycin-2 -deoxyriboside with beta-configuration ([(18)F]FAZDR, [(18)F]-beta-8) to mimic nucleosides more closely and comparatively evaluate it versus [(18)F]FAZA. METHODS: Precursor and cold standards for [(18)F]FAZDR were synthesized from methyl 2-deoxy-d-ribofuranosides alpha- and beta-1 in 6 steps yielding precursors alpha- and beta-5. beta-5 was radiolabeled in a GE TRACERlab FXF-N synthesizer in DMSO and deprotected with NH4OH to give [(18)F]FAZDR ([(18)F]-beta-8). [(18)F]FAZA or [(18)F]FAZDR was injected in BALB/c mice bearing CT26 colon carcinoma xenografts, PET scans (10min) were performed after 1, 2 and 3h post injection (p.i.). On a subset of mice injected with [(18)F]FAZDR, we analyzed biodistribution. RESULTS: [(18)F]FAZDR was obtained in non-corrected yields of 10.9+/-2.4% (9.1+/-2.2GBq, n=4) 60min EOB, with radiochemical purity >98% and specific activity >50GBq/mumol. Small animal PET imaging showed a decrease in uptake over time for both [(18)F]FAZDR (1h p.i.: 0.56+/-0.22% ID/cc, 3h: 0.17+/-0.08% ID/cc, n=9) and [(18)F]FAZA (1h: 1.95+/-0.59% ID/cc, 3h: 0.87+/-0.55% ID/cc), whereas T/M ratios were significantly higher for [(18)F]FAZDR at 1h (2.76) compared to [(18)F]FAZA (1.69, P<0.001), 3h p.i. ratios showed no significant difference. Moreover, [(18)F]FAZDR showed an inverse correlation between tracer uptake in carcinomas and oxygen breathing, while muscle tissue uptake was not affected by switching from air to oxygen. CONCLUSIONS: First PET imaging results with [(18)F]FAZDR showed advantages over [(18)F]FAZA regarding higher tumor contrast at earlier time points p.i. Availability of precursor and cold fluoro standard together with high output radiosynthesis will allow for a more detailed quantitative evaluation of [(18)F]FAZDR, especially with regard to mechanistic studies whether active transport processes are involved, compared to passive diffusion as observed for [(18)F]FAZA.


