SulforaphaneCAS# 4478-93-7 |
- L-Sulforaphane
Catalog No.:BCN8449
CAS No.:142825-10-3
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4478-93-7 | SDF | Download SDF |
| PubChem ID | 5350 | Appearance | White powder |
| Formula | C6H11NOS2 | M.Wt | 177.29 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 62.5 mg/mL (352.53 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1-isothiocyanato-4-methylsulfinylbutane | ||
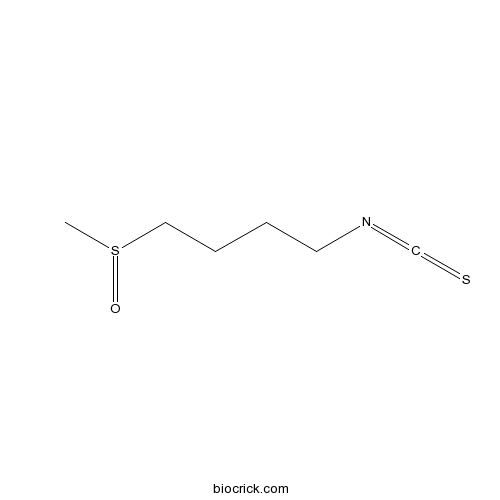
| SMILES | CS(=O)CCCCN=C=S | ||
| Standard InChIKey | SUVMJBTUFCVSAD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H11NOS2/c1-10(8)5-3-2-4-7-6-9/h2-5H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Sulforaphane is a cruciferous vegetable-derived isothiocyanate with promising chemopreventive and therapeutic activities. 2. Sulforaphane might exert a significant neuroprotective effect on cholinergic deficit and cognitive impairment. 3. Sulforaphane has anti-inflammatory effect, at least ,in part,associated with interfering with the NF-κB pathway. 4. Sulforaphane inhibits TPA-induced NF-κB activation and COX-2 expression in MCF-10A cells by blocking two distinct signaling pathways mediated by ERK1/2-IKKα and NAK-IKKβ. 5. Sulforaphane has antitumor effects gainst bladder cancer cells through an ROS-mediated intrinsic apoptotic pathway, and suggest that ER stress and Nrf2 may represent strategic targets for Sulforaphane-induced apoptosis. |
| Targets | Akt | ERK | TNF-α | p65 | NF-kB | IkB | COX | Nrf2 | HO-1 | Bcl-2/Bax | Caspase | AChR | IKK |

Sulforaphane Dilution Calculator

Sulforaphane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6405 mL | 28.2024 mL | 56.4048 mL | 112.8095 mL | 141.0119 mL |
| 5 mM | 1.1281 mL | 5.6405 mL | 11.281 mL | 22.5619 mL | 28.2024 mL |
| 10 mM | 0.564 mL | 2.8202 mL | 5.6405 mL | 11.281 mL | 14.1012 mL |
| 50 mM | 0.1128 mL | 0.564 mL | 1.1281 mL | 2.2562 mL | 2.8202 mL |
| 100 mM | 0.0564 mL | 0.282 mL | 0.564 mL | 1.1281 mL | 1.4101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulforaphane is an isothiocyanate present naturally in widely consumed vegetables; has shown anticancer and cardioprotective activities.
In Vitro:Sulforaphane induces a cell cycle arrest in a dose-dependent manner, followed by cell death. This sulforaphane-induced cell cycle arrest was correlated with an increased expression of cyclins A and B1. Sulforaphane induces cell death via an apoptotic process. Sulforaphane inhibits the reinitiation of growth and diminishes cellular viability in quiescent colon carcinoma cells (HT29) and has a lower toxicity on differentiated CaCo2 cells[1]. Pre-treatment of H9c2 rat myoblasts with sulforaphane decreases the apoptotic cell number and the expression of pro-apoptotic proteins (Bax, caspase-3 and cytochrome c), as well as the doxorubicin-induced increase in mitochondrial membrane potential. Furthermore, sulforaphane increases the mRNA and protein expression of heme oxygenase-1, which consequently reduces the levels of reactive oxygen species (ROS, measured using MitoSOX Red reagent) in the mitochondria which are induced by doxorubicin[2].
In Vivo:Sulforaphane can block the formation of ammary tumors in Sprague-Dawley rats treated with single doses of 9,10-dimethyl-1,2-benzanthracene. Administration of sulforaphane reduces the incidence, multiplicity, and weights and delays the development of the mammary tumors evoked by a single dose of DMBA in female Sprague-Dawley rats[3].
References:
[1]. Gamet-Payrastre L, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000 Mar 1;60(5):1426-33.
[2]. Li B, et al. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int J Mol Med. 2015 Jul;36(1):53-64.
[3]. Zhang Y, et al. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornylisothiocyanates. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3147-50.
- Ruixianglangdusu B
Catalog No.:BCN6869
CAS No.:447454-49-1
- RLLFT-NH2
Catalog No.:BCC3954
CAS No.:447408-68-6
- Angiotensin II human
Catalog No.:BCC4087
CAS No.:4474-91-3
- RepSox
Catalog No.:BCC1887
CAS No.:446859-33-2
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one
Catalog No.:BCC8646
CAS No.:446292-10-0
- YM 230888
Catalog No.:BCC5956
CAS No.:446257-23-4
- 2,4,5-Trimethoxybenzaldehyde
Catalog No.:BCN5498
CAS No.:4460-86-0
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Homoeriodictyol
Catalog No.:BCN6804
CAS No.:446-71-9
- BMS CCR2 22
Catalog No.:BCC7572
CAS No.:445479-97-0
- SGS 518 oxalate
Catalog No.:BCC7750
CAS No.:445441-27-0
- Betulonic acid
Catalog No.:BCN5500
CAS No.:4481-62-3
- NS 1643
Catalog No.:BCC7552
CAS No.:448895-37-2
- WY 45233 succinate
Catalog No.:BCC6125
CAS No.:448904-47-0
- 3-(4-Methoxyphenyl)-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN1440
CAS No.:448905-82-6
- ITE
Catalog No.:BCC3902
CAS No.:448906-42-1
- Quercetin 3-O-[2-O-(6-O-E-feruloyl)-beta-D-glucopyranosyl]-beta-D-galactopyranoside
Catalog No.:BCN1439
CAS No.:448948-20-7
- 4',4''-Dihydroxyanigorootin
Catalog No.:BCN7153
CAS No.:448949-11-9
- Rubinaphthin A
Catalog No.:BCN3511
CAS No.:448962-05-8
- Indaconitine
Catalog No.:BCN6259
CAS No.:4491-19-4
- 9-O-Acetyl-fargesol
Catalog No.:BCN8216
CAS No.:449172-61-6
- VPC 23019
Catalog No.:BCC7883
CAS No.:449173-19-7
- (+)-S-Myricanol glucoside
Catalog No.:BCN5501
CAS No.:449729-89-9
Sulforaphane down-regulates SKP2 to stabilize p27(KIP1) for inducing antiproliferation in human colon adenocarcinoma cells.[Pubmed:25070589]
J Biosci Bioeng. 2015 Jan;119(1):35-42.
Sulforaphane is a cruciferous vegetable-derived isothiocyanate with promising chemopreventive and therapeutic activities. Induction of proliferation arrest and apoptosis principally contribute to Sulforaphane's anticancer activity, but the precise molecular mechanisms remain elusive. The oncoprotein SKP2 is a key component of the SKP1-CULLIN1-F-box (SCF) E3 ligase complex and is responsible for directing SCF-mediated degradation of cyclin-dependent kinase inhibitor p27(KIP1) to promote cell proliferation. We herein provide the first evidence supporting the critical involvement of the SKP2-p27(KIP1) axis in Sulforaphane-induced antiproliferation in various human colon adenocarcinoma cell lines. Specifically, Sulforaphane markedly suppressed the levels of bromodeoxyuridine (BrdU) incorporation and clonogenicity in all tested cell lines, illustrating the antiproliferative effect of Sulforaphane. Of note, Sulforaphane-induced antiproliferation was accompanied with down-regulation of SKP2, leading to the stabilization and thus up-regulation of p27(KIP1). Additionally, Sulforaphane was found to down-regulate SKP2 mainly through transcriptional repression, as Sulforaphane lowered SKP2 mRNA expression and the SKP2 promoter activity. Furthermore, Sulforaphane treatment led to the activation of both AKT and ERK, thus ruling out the possibility that Sulforaphane down-regulates SKP2 by inhibiting AKT or ERK. Notably, Sulforaphane-elicited suppression of BrdU incorporation and clonogenicity were significantly rescued in the context of SKP2 overexpression or p27(KIP1) depletion, therefore highlighting the important role of SKP2 down-regulation and the ensuing stabilization of p27(KIP1) in Sulforaphane-induced antiproliferation. Collectively, these data expand our molecular understanding about how Sulforaphane elicits proliferation arrest, but also implicate the application of Sulforaphane in therapeutic modalities targeting SKP2.
Sulforaphane inhibits phorbol ester-stimulated IKK-NF-kappaB signaling and COX-2 expression in human mammary epithelial cells by targeting NF-kappaB activating kinase and ERK.[Pubmed:24747121]
Cancer Lett. 2014 Aug 28;351(1):41-9.
Sulforaphane, an isothiocyanate present in cruciferous vegetables, has been reported to possess anti-inflammatory and cancer chemopreventive properties. However, the molecular mechanisms by which Sulforaphane suppresses inflammation and carcinogenesis are yet to be fully elucidated. Since the aberrant expression of cyclooxygenase-2 (COX-2) links inflammation and cancer, the present study was aimed to elucidate the mechanisms by which Sulforaphane modulates COX-2 overexpression in human mammary epithelial (MCF-10A) cells stimulated with a prototypic tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA). Treatment of MCF-10A cells with Sulforaphane significantly inhibited TPA-induced expression of COX-2 protein and its mRNA transcript. Transient transfection of cells with deletion mutant constructs of COX-2 promoter revealed that the transcription factor nuclear factor-kappaB (NF-kappaB) plays a key role in TPA-induced COX-2 expression in MCF-10A cells. Pretreatment with Sulforaphane significantly attenuated nuclear localization, DNA binding and the transcriptional activity of NF-kappaB through inhibition of phosphorylation and subsequent degradation of IkappaBalpha in MCF-10A cells stimulated with TPA. Sulforaphane also attenuated TPA-induced activation of IkappaB kinases (IKK), NF-kappaB-activating kinase (NAK) and extracellular signal-regulated kinase-1/2 (ERK1/2). Pharmacological inhibition of IKK or transient transfection of cells with dominant-negative mutant forms of this kinase abrogated TPA-induced NF-kappaB activation and COX-2 expression. In addition, the blockade of ERK1/2 activation negated the catalytic activity of IKKalpha, but not that of IKKbeta, whereas silencing NAK by specific siRNA abrogated the IKKbeta activity in TPA-treated cells. Taken together, Sulforaphane inhibits TPA-induced NF-kappaB activation and COX-2 expression in MCF-10A cells by blocking two distinct signaling pathways mediated by ERK1/2-IKKalpha and NAK-IKKbeta.
Sulforaphane reduces vascular inflammation in mice and prevents TNF-alpha-induced monocyte adhesion to primary endothelial cells through interfering with the NF-kappaB pathway.[Pubmed:24880493]
J Nutr Biochem. 2014 Aug;25(8):824-33.
Sulforaphane, a naturally occurring isothiocyanate present in cruciferous vegetables, has received wide attention for its potential to improve vascular function in vitro. However, its effect in vivo and the molecular mechanism of Sulforaphane at physiological concentrations remain unclear. Here, we report that a Sulforaphane concentration as low as 0.5 muM significantly inhibited tumor necrosis factor-alpha (TNF-alpha)-induced adhesion of monocytes to human umbilical vein endothelial cells, a key event in the pathogenesis of atherosclerosis both in static and under flow conditions. Such physiological concentrations of Sulforaphane also significantly suppressed TNF-alpha-induced production of monocyte chemotactic protein-1 and adhesion molecules including soluble vascular adhesion molecule-1 and soluble E-selectin, key mediators in the regulation of enhanced endothelial cell-monocyte interaction. Furthermore, Sulforaphane inhibited TNF-alpha-induced nuclear factor (NF)-kappaB transcriptional activity, Inhibitor of NF-kappaB alpha (IkappaBalpha) degradation and subsequent NF-kappaB p65 nuclear translocation in endothelial cells, suggesting that Sulforaphane can inhibit inflammation by suppressing NF-kappaB signaling. In an animal study, Sulforaphane (300 ppm) in a mouse diet significantly abolished TNF-alpha-increased ex vivo monocyte adhesion and circulating adhesion molecules and chemokines in C57BL/6 mice. Histology showed that Sulforaphane treatment significantly prevented the eruption of endothelial lining in the intima layer of the aorta and preserved elastin fibers' delicate organization, as shown by Verhoeff-van Gieson staining. Immunohistochemistry studies showed that Sulforaphane treatment also reduced vascular adhesion molecule-1 and monocyte-derived F4/80-positive macrophages in the aorta of TNF-alpha-treated mice. In conclusion, Sulforaphane at physiological concentrations protects against TNF-alpha-induced vascular endothelial inflammation, in both in vitro and in vivo models. This anti-inflammatory effect of Sulforaphane may be, at least in part, associated with interfering with the NF-kappaB pathway.
Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: the involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway.[Pubmed:24993616]
Int J Oncol. 2014 Oct;45(4):1497-506.
Sulforaphane, a naturally occurring isothiocyanate found in cruciferous vegetables, has received a great deal of attention because of its ability to inhibit cell proliferation and induce apoptosis in cancer cells. In this study, we investigated the anticancer activity of Sulforaphane in the T24 human bladder cancer line, and explored its molecular mechanism of action. Our results showed that treatment with Sulforaphane inhibited cell viability and induced apoptosis in T24 cells in a concentration-dependent manner. Sulforaphane-induced apoptosis was associated with mitochondria dysfunction, cytochrome c release and Bcl-2/Bax dysregulation. Furthermore, the increased activity of caspase-9 and -3, but not caspase-8, was accompanied by the cleavage of poly ADP-ribose polymerase, indicating the involvement of the mitochondria-mediated intrinsic apoptotic pathway. Concomitant with these changes, Sulforaphane triggered reactive oxygen species (ROS) generation, which, along with the blockage of Sulforaphane-induced loss of mitochondrial membrane potential and apoptosis, was strongly attenuated by the ROS scavenger N-acetyl-L-cysteine. Furthermore, Sulforaphane was observed to activate endoplasmic reticulum (ER) stress and the nuclear factor-E2-related factor-2 (Nrf2) signaling pathway, as demonstrated by the upregulation of ER stressrelated proteins, including glucose-regulated protein 78 and C/EBP-homologous protein, and the accumulation of phosphorylated Nrf2 proteins in the nucleus and induction of heme oxygenase-1 expression, respectively. Taken together, these results demonstrate that Sulforaphane has antitumor effects against bladder cancer cells through an ROS-mediated intrinsic apoptotic pathway, and suggest that ER stress and Nrf2 may represent strategic targets for Sulforaphane-induced apoptosis.
Sulforaphane alleviates scopolamine-induced memory impairment in mice.[Pubmed:24836869]
Pharmacol Res. 2014 Jul;85:23-32.
Sulforaphane, an organosulfur compound present in cruciferous vegetables, has been shown to exert neuroprotective effects in experimental in vitro and in vivo models of neurodegeneration. To determine whether Sulforaphane can preserve cognitive function, we examined its effects on scopolamine-induced memory impairment in mice using the Morris water maze test. Sulforaphane (10 or 50mg/kg) was administered to C57BL/6 mice by oral gavage for 14 days (days 1-14), and memory impairment was induced by intraperitoneal injection of scopolamine (1mg/kg) for 7 days (days 8-14). Mice that received scopolamine alone showed impaired learning and memory retention and considerably decreased cholinergic system reactivity in the hippocampus and frontal cortex, as indicated by a decreased acetylcholine (ACh) level and an increased acetylcholinesterase (AChE) activity. Sulforaphane significantly attenuated the scopolamine-induced memory impairment and improved cholinergic system reactivity, as indicated by an increased ACh level, decreased AChE activity, and increased choline acetyltransferase (ChAT) expression in the hippocampus and frontal cortex. These effects of Sulforaphane on cholinergic system reactivity were confirmed in vitro. Sulforaphane (10 or 20muM) increased the ACh level, decreased the AChE activity, and increased ChAT expression in scopolamine-treated primary cortical neurons. These observations suggest that Sulforaphane might exert a significant neuroprotective effect on cholinergic deficit and cognitive impairment.


