NS 1643KV11.1 (hERG) channel activator; antiarrhythmic CAS# 448895-37-2 |
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 448895-37-2 | SDF | Download SDF |
| PubChem ID | 10177784 | Appearance | Powder |
| Formula | C15H10F6N2O3 | M.Wt | 380.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (262.99 mM) *"≥" means soluble, but saturation unknown. | ||
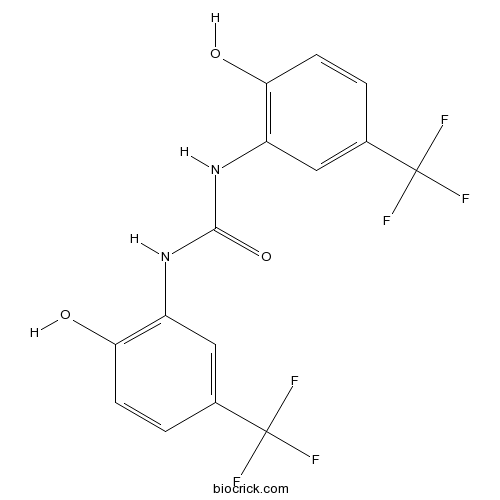
| Chemical Name | 1,3-bis[2-hydroxy-5-(trifluoromethyl)phenyl]urea | ||
| SMILES | C1=CC(=C(C=C1C(F)(F)F)NC(=O)NC2=C(C=CC(=C2)C(F)(F)F)O)O | ||
| Standard InChIKey | NJFVQMRYJZHGME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10F6N2O3/c16-14(17,18)7-1-3-11(24)9(5-7)22-13(26)23-10-6-8(15(19,20)21)2-4-12(10)25/h1-6,24-25H,(H2,22,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Human ether-a-go-go related gene (hERG) KV11.1 channel activator (EC50 = 10.5 μM). Exhibits different molecular mechanisms of action at KV11.1 (hERG1) and KV11.2 (hERG2) channels. Displays antiarrhythmic activity in vitro. |

NS 1643 Dilution Calculator

NS 1643 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6299 mL | 13.1496 mL | 26.2992 mL | 52.5984 mL | 65.7479 mL |
| 5 mM | 0.526 mL | 2.6299 mL | 5.2598 mL | 10.5197 mL | 13.1496 mL |
| 10 mM | 0.263 mL | 1.315 mL | 2.6299 mL | 5.2598 mL | 6.5748 mL |
| 50 mM | 0.0526 mL | 0.263 mL | 0.526 mL | 1.052 mL | 1.315 mL |
| 100 mM | 0.0263 mL | 0.1315 mL | 0.263 mL | 0.526 mL | 0.6575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NS1643 is a potent human ether-a-go-go related gene (hERG) KV11.1 channel activator (EC50 = 10.5 μM). IC50 value: Target: HERG activator in vitro: NS1643 enhanced the magnitude of wild-type hERG current in a concentration- and voltage-dependent manner with an EC(50) of 10.4 microM at -10 mV. The fully activated current-voltage relationship revealed that the drug increased outward but not inward currents, consistent with altered inactivation gating. NS1643 shifted the voltage dependence of inactivation by +21 mV at 10 microM and +35 mV at 30 microM, but it did not alter the voltage dependence of activation of hERG channels [1]. In Xenopus laevis oocytes, NS1643 increased both steady-state and tail current at all voltages tested. The EC(50) value for HERG channel activation was 10.5 microM [2]. NS1643 also activates the ERG2 channel; however, the molecular mechanism of the activation differs between the ERG1 and ERG2 channels. For ERG2, NS1643 causes a left-ward shift of the activation curve, a faster time-constant of activation and a slower time-constant of inactivation as well as an increased relative importance for the fast component of deactivation to the total deactivation. In contrast, for ERG1, NS1643 causes a right-ward shift in the voltage-dependent release from inactivation but does not affect time-constants of deactivation [3].
References:
[1]. Casis O, et al. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol. 2006 Feb;69(2):658-65.
[2]. Hansen RS, et al. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643). Mol Pharmacol. 2006 Jan;69(1):266-77.
[3]. Elmedyb P, et al. Activation of ERG2 potassium channels by the diphenylurea NS1643. Neuropharmacology. 2007 Aug;53(2):283-94.
- Betulonic acid
Catalog No.:BCN5500
CAS No.:4481-62-3
- Sulforaphane
Catalog No.:BCN2349
CAS No.:4478-93-7
- Ruixianglangdusu B
Catalog No.:BCN6869
CAS No.:447454-49-1
- RLLFT-NH2
Catalog No.:BCC3954
CAS No.:447408-68-6
- Angiotensin II human
Catalog No.:BCC4087
CAS No.:4474-91-3
- RepSox
Catalog No.:BCC1887
CAS No.:446859-33-2
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one
Catalog No.:BCC8646
CAS No.:446292-10-0
- YM 230888
Catalog No.:BCC5956
CAS No.:446257-23-4
- 2,4,5-Trimethoxybenzaldehyde
Catalog No.:BCN5498
CAS No.:4460-86-0
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Homoeriodictyol
Catalog No.:BCN6804
CAS No.:446-71-9
- WY 45233 succinate
Catalog No.:BCC6125
CAS No.:448904-47-0
- 3-(4-Methoxyphenyl)-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN1440
CAS No.:448905-82-6
- ITE
Catalog No.:BCC3902
CAS No.:448906-42-1
- Quercetin 3-O-[2-O-(6-O-E-feruloyl)-beta-D-glucopyranosyl]-beta-D-galactopyranoside
Catalog No.:BCN1439
CAS No.:448948-20-7
- 4',4''-Dihydroxyanigorootin
Catalog No.:BCN7153
CAS No.:448949-11-9
- Rubinaphthin A
Catalog No.:BCN3511
CAS No.:448962-05-8
- Indaconitine
Catalog No.:BCN6259
CAS No.:4491-19-4
- 9-O-Acetyl-fargesol
Catalog No.:BCN8216
CAS No.:449172-61-6
- VPC 23019
Catalog No.:BCC7883
CAS No.:449173-19-7
- (+)-S-Myricanol glucoside
Catalog No.:BCN5501
CAS No.:449729-89-9
- Specneuzhenide
Catalog No.:BCC9151
CAS No.:449733-84-0
- FAK Inhibitor 14
Catalog No.:BCC7683
CAS No.:4506-66-5
Activation of ERG2 potassium channels by the diphenylurea NS1643.[Pubmed:17610913]
Neuropharmacology. 2007 Aug;53(2):283-94.
Three members of the ERG potassium channel family have been described (ERG1-3 or Kv 11.1-3). ERG1 is by far the best characterized subtype and it constitutes the molecular component of the cardiac I(Kr) current. All three channel subtypes are expressed in neurons but their function remains unclear. The lack of functional information is at least partly due to the lack of specific pharmacological tools. The compound NS1643 has earlier been reported as an ERG1 channel activator. We found that NS1643 also activates the ERG2 channel; however, the molecular mechanism of the activation differs between the ERG1 and ERG2 channels. This is surprising since ERG1 and ERG2 channels have very similar biophysical and structural characteristics. For ERG2, NS1643 causes a left-ward shift of the activation curve, a faster time-constant of activation and a slower time-constant of inactivation as well as an increased relative importance for the fast component of deactivation to the total deactivation. In contrast, for ERG1, NS1643 causes a right-ward shift in the voltage-dependent release from inactivation but does not affect time-constants of deactivation. Because of these differences in the responses of ERG1 and ERG2 to NS1643, NS1643 can be used as a pharmacological tool to address ERG channel function. It may be useful for revealing physiological functions of ERG channels in neuronal tissue as well as to elucidate the structure-function relationships of the ERG channels.
Mechanism of action of a novel human ether-a-go-go-related gene channel activator.[Pubmed:16284303]
Mol Pharmacol. 2006 Feb;69(2):658-65.
1,3-Bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) is a newly discovered activator of human ether-a-go-go-related gene (hERG) K(+) channels. Here, we characterize the effects of this compound on cloned hERG channels heterologously expressed in Xenopus laevis oocytes. When assessed with 2-s depolarizations, NS1643 enhanced the magnitude of wild-type hERG current in a concentration- and voltage-dependent manner with an EC(50) of 10.4 microM at -10 mV. The fully activated current-voltage relationship revealed that the drug increased outward but not inward currents, consistent with altered inactivation gating. NS1643 shifted the voltage dependence of inactivation by +21 mV at 10 microM and +35 mV at 30 microM, but it did not alter the voltage dependence of activation of hERG channels. The effects of the drug on three inactivation-deficient hERG mutant channels (S620T, S631A, and G628C/S631C) were determined. In the absence of channel inactivation, NS1643 did not enhance hERG current magnitude. The agonist activity of NS1643 was facilitated by mutations (F656 to Val, Met, or Thr) that are known to greatly attenuate channel inhibition by hERG blockers. We conclude that NS1643 is a partial agonist of hERG channels and that the mechanism of activation is reduced channel inactivation.
Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643).[Pubmed:16219910]
Mol Pharmacol. 2006 Jan;69(1):266-77.
The cardiac action potential is generated by a concerted action of different ion channels and transporters. Dysfunction of any of these membrane proteins can give rise to cardiac arrhythmias, which is particularly true for the repolarizing potassium channels. We suggest that an increased repolarization current could be a new antiarrhythmic principle, because it possibly would attenuate afterdepolarizations, ischemic leak currents, and reentry phenomena. Repolarization of the cardiac myocytes is crucially dependent on the late rapid delayed rectifier current (I(Kr)) conducted by ether-a-go-go-related gene (ERG) potassium channels. We have developed the diphenylurea compound 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) and tested whether this small organic molecule could increase the activity of human ERG (HERG) channels expressed heterologously. In Xenopus laevis oocytes, NS1643 increased both steady-state and tail current at all voltages tested. The EC(50) value for HERG channel activation was 10.5 microM. These results were reproduced on HERG channels expressed in mammalian human embryonic kidney 293 cells. In guinea pig cardiomyocytes, studied by patch clamp, application of 10 microM NS1643 activated I(Kr) and significantly decreased the action potential duration to 65% of the control values. The effect could be reverted by application of the specific HERG channel inhibitor 4'-[[1-[2-(6-methyl-2-pyridyl)ethyl]-4-piperidinyl]carbonyl]-methanesulfonanilide (E-4031) at 100 nM. Application of NS1643 also resulted in a prolonged postrepolarization refractory time. Finally, cardiomyocytes exposed to NS1643 resisted reactivation by small depolarizing currents mimicking early afterdepolarizations. In conclusion, HERG channel activation by small molecules such as NS1643 increases the repolarization reserve and presents an interesting new antiarrhythmic approach.


