MelphalanDNA alkylating agent CAS# 148-82-3 |
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

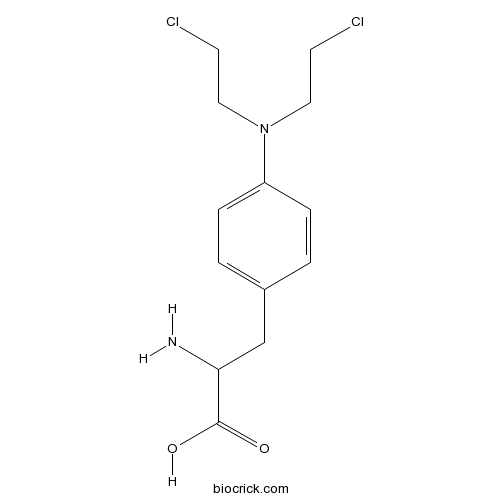
| Cas No. | 148-82-3 | SDF | Download SDF |
| PubChem ID | 4053 | Appearance | Powder |
| Formula | C13H18Cl2N2O2 | M.Wt | 305.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-PAM | ||
| Solubility | DMSO : 5 mg/mL (16.38 mM; Need ultrasonic) H2O : 4 mg/mL (13.11 mM; ultrasonic and adjust pH to 2 with HCl) | ||
| Chemical Name | 2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoic acid | ||
| SMILES | C1=CC(=CC=C1CC(C(=O)O)N)N(CCCl)CCCl | ||
| Standard InChIKey | SGDBTWWWUNNDEQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H18Cl2N2O2/c14-5-7-17(8-6-15)11-3-1-10(2-4-11)9-12(16)13(18)19/h1-4,12H,5-9,16H2,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | DNA alkylating agent; induces cytotoxicity through the formation of stable interstrand and intrastrand crosslinks within DNA. Inhibits growth of PC-3 cells (IC50 values are 0.074 and 0.77 μg/ml for sequential dosing and single dosing respectively). |

Melphalan Dilution Calculator

Melphalan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2765 mL | 16.3827 mL | 32.7654 mL | 65.5308 mL | 81.9135 mL |
| 5 mM | 0.6553 mL | 3.2765 mL | 6.5531 mL | 13.1062 mL | 16.3827 mL |
| 10 mM | 0.3277 mL | 1.6383 mL | 3.2765 mL | 6.5531 mL | 8.1913 mL |
| 50 mM | 0.0655 mL | 0.3277 mL | 0.6553 mL | 1.3106 mL | 1.6383 mL |
| 100 mM | 0.0328 mL | 0.1638 mL | 0.3277 mL | 0.6553 mL | 0.8191 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Melphalan is a DNA alkylating agent and inhibits DNA and RNA synthesis [1].
DNA alkylating agent attaches the alkyl group to the guanine base of DNA and inhibits DNA and RNA synthesis, which is necessary for cells to survive.
In PC-3 cells, melphalan inhibited cells growth with IC50 values of 0.074 μg/ml and 0.77 μg/ml for sequential dosing and single dosing, respectively, which suggested the sequential dosing was more effective [1].
In 12 patients with androgen-independent prostate cancer, melphalan provided some clinical benefits with manageable toxicity and the median survival was 23 weeks [1]. In 381 myeloma patients who received melphalan-based autologous stem cell transplant (Mel-ASCT), melphalan (200 mg/m2 body surface area (BSA)) led to oral mucositis (OM) in 75% of patients. And OM was severe in 21% patients. Patients with renal dysfunction have the greatest risk for severe OM when received a high mg/kg melphalan dose [2].
References:
[1]. Mougenot P, Bressolle F, Culine S, et al. In vitro cytotoxic effect of melphalan and pilot phase II study in hormone-refractory prostate cancer. Anticancer Res, 2006, 26(3B): 2197-2203.
[2]. Grazziutti ML, Dong L, Miceli MH, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant, 2006, 38(7): 501-506.
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
Phase II Study of Yttrium-90 Ibritumomab Tiuxetan Plus High-Dose BCNU, Etoposide, Cytarabine, and Melphalan for Non-Hodgkin Lymphoma: The Role of Histology.[Pubmed:28267593]
Biol Blood Marrow Transplant. 2017 Jun;23(6):922-929.
Standard-dose (90)yttrium-ibritumomab tiuxetan (.4 mci/kg) together with high-dose BEAM (BCNU, etoposide, cytarabine, and Melphalan) (Z-BEAM) has been shown to be a well-tolerated autologous hematopoietic stem cell transplantation preparative regimen for non-Hodgkin lymphoma. We report the outcomes of a single-center, single-arm phase II trial of Z-BEAM conditioning in high-risk CD20(+) non-Hodgkin lymphoma histologic strata: diffuse large B cell (DLBCL), mantle cell, follicular, and transformed. Robust overall survival and notably low nonrelapse mortality rates (.9% at day +100 for the entire cohort), with few short- and long-term toxicities, confirm the safety and tolerability of the regimen. In addition, despite a high proportion of induction failure patients (46%), the promising response and progression-free survival (PFS) rates seen in DLBCL (3-year PFS: 71%; 95% confidence interval, 55 to 82%), support the premise that the Z-BEAM regimen is particularly effective in this histologic subtype. The role of Z-BEAM in other strata is less clear in the context of the emergence of novel agents.
A Phase I Trial of High-Dose Lenalidomide and Melphalan as Conditioning for Autologous Stem Cell Transplantation in Relapsed or Refractory Multiple Myeloma.[Pubmed:28285081]
Biol Blood Marrow Transplant. 2017 Jun;23(6):930-937.
Autologous stem cell transplantation (ASCT) conditioned with high-dose chemotherapy has long been established as the standard of care for eligible patients with newly diagnosed multiple myeloma. Despite recent therapeutic advances, high-dose Melphalan (HDM) remains the chemotherapy regimen of choice in this setting. Lenalidomide (LEN) in combination with low-dose dexamethasone is recognized as a standard of care for patients with relapsed or refractory multiple myeloma (RRMM), and there is growing support for the administration of LEN as maintenance therapy post-ASCT. In view of the above, the present phase I clinical trial was designed to evaluate the safety and tolerability of high-dose LEN (HDLEN) in patients with RRMM, and to determine the maximum tolerated dose of HDLEN when added to HDM before ASCT. Despite administering HDLEN at doses of up to 350 mg/day, the maximum tolerated dose could not be determined, owing to an insufficient number of dose-limiting toxicities in the 21 patients enrolled in the trial. Conditioning with HDLEN plus HDM was associated with a favorable tolerability profile. Adverse events following ASCT were as expected with HDM. Median progression-free and overall survival were 10 months and 22 months, respectively, in this population of heavily pretreated patients. Our findings suggest that HDLEN in combination with HDM may offer significant potential as a conditioning regimen before ASCT in patients with RRMM. These preliminary findings are now being evaluated further in an ongoing phase II clinical trial.
Long-term follow-up of patients with multiple myeloma treated with total body irradiation-Melphalan conditioning.[Pubmed:28370630]
Eur J Haematol. 2017 Jul;99(1):56-59.
BACKGROUND AND OBJECTIVES: Since a study published in 2002 showed a survival advantage of Melphalan-only conditioning for stem cell transplantation (HSCT) over Melphalan-total body irradiation (mel-TBI) in patients with multiple myeloma (MM), most centers abandoned mel-TBI. Mel-TBI causes more early toxicity and is more complicated to administer, but we speculated it may result in longer term survival with radiation as an independent treatment modality. Therefore, we analyzed the long-term outcome of patients with MM who received mel-TBI as part of conditioning at our center. PATIENTS AND METHODS: From 1995 to 2013, 50 patients with MM underwent autologous HSCT at Tulane University Medical Center using mel-TBI conditioning. We used Kaplan-Meier survival analysis and compared our patients with data available from the Louisiana Tumor Registry. RESULTS: The mean survival of our patients was 70.98 months from time of transplant and 84.2 months from time of initial diagnosis. No differences were observed according to gender, ethnicity, or age at transplant. The expected median survival in a population-based registry (matched for age and year of treatment) was 27 months (P<.001). CONCLUSIONS: Total body irradiation in conjunction with Melphalan as conditioning is feasible and can lead to long-term survival. More research is necessary to determine which patients benefit most. Mel-TBI should also be explored in conjunction with immunotherapy.
Acute Hemorrhagic Retinopathy following Intravitreal Melphalan Injection for Retinoblastoma: A Report of Two Cases and Technical Modifications to Enhance the Prevention of Retinal Toxicity.[Pubmed:28275601]
Ocul Oncol Pathol. 2017 Jan;3(1):34-40.
AIMS: To report the occurrence of acute hemorrhagic retinopathy following intravitreal Melphalan injection for retinoblastoma. METHODS: This is a retrospective case series of 2 patients with retinoblastoma treated with intravitreal Melphalan for vitreous seeding who developed acute hemorrhagic retinopathy. RESULTS: Patient 1 is a 6-month-old female with bilateral retinoblastoma (Group D right eye and Group B left eye) treated with 4 cycles of systemic chemotherapy and 2 intravitreal Melphalan injections in each eye. Patient 2 is a 10-month-old male with unilateral Group D retinoblastoma treated with 6 cycles of systemic chemotherapy and 2 injections of intravitreal Melphalan. At the 1-week follow-up after the second injection, both patients had an acute hemorrhagic retinopathy that resulted in chorioretinal toxicity with a sharp demarcation line between the normal and abnormal retina. At the last follow-up (22 and 12 months, respectively), there was total tumor control and resolution of vitreous seeding in both patients. CONCLUSIONS: Although intravitreal Melphalan injection is effective for vitreous seeding in eyes with retinoblastoma, acute hemorrhagic retinopathy and diffuse chorioretinal atrophy is a possible complication of this treatment modality. Given the clinical findings observed in these patients, the development of this retinal toxicity most likely results from a retrohyaloid overdose. Consequently we suggest preventive measures aimed at limiting the risk of retrohyaloid injection.
In vitro cytotoxic effect of melphalan and pilot phase II study in hormone-refractory prostate cancer.[Pubmed:16821586]
Anticancer Res. 2006 May-Jun;26(3B):2197-203.
The objective of this study was to determine the impact of single versus sequential exposure to Melphalan on the proliferation of an androgen-independent prostate cell line, PC-3, and to report the results of a pilot phase II study. For exposure to a single bolus dose, the doses were added at the start of the study and cell culture was continued for 96 h. For sequential exposure, 1/9 of the dose was added every 1.5 h over 12 h, followed by cell culture for 84 h. Cell growth inhibition was determined by the MTT assay. The clinical study was carried out on 14 patients with advanced prostate cancer. Melphalan was infused over a 24-h period. The sequential-dose schedule was more effective than the single-dose exposure, IC50 values: 0.074 versus 0.77/microg/ml. Out of the 14 patients (42 courses) enrolled into the study, two patients were removed within the first 2 weeks because of rapid disease progression. The toxicity profile did not differ greatly from that reported after a 1-h infusion. Four PR and two SD were observed. The median survival of the twelve patients was 23 weeks. Melphalan administered over a 24-h period to patients with androgen-independent prostate cancer appeared to provide some clinical benefits with manageable toxicity.
Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence.[Pubmed:9092631]
Nucleic Acids Res. 1997 Mar 15;25(6):1211-8.
Previous work showed that Melphalan-induced mutations in the aprt gene of CHO cells are primarily transversions and occur preferentially at G-G-C sequences, which are potential sites for various bifunctional alkylations involving guanine N-7. To identify the DNA lesion(s) which may be responsible for these mutations, an end-labeled DNA duplex containing a frequent site of Melphalan-induced mutation in the aprt gene was treated with Melphalan, mechlorethamine or phosphoramide mustard. The sequence specificity and kinetics of formation of both interstrand and intrastrand crosslinks were determined. All mustards selectively formed two base-staggered interstrand crosslinks between the 5'G and the G opposite C in the 5'G-G-C sequence. Secondary alkylation was much slower for Melphalan than for the other mustards and the resulting crosslink was more stable. Mechlorethamine and phosphoramide mustard induced intrastrand crosslinks between the two contiguous Gs in the G-G-C sequence in double-stranded DNA, but Melphalan did not. Molecular dynamic simulations provided a structural explanation for this difference, in that the monofunctionally bound intermediates of mechlorethamine and phosphoramide mustard assumed thermodynamically stable conformations with the second arm in a position appropriate for intrastrand crosslink formation, while the corresponding Melphalan monoadduct did not.


