Santacruzamate A (CAY10683)HDAC inhibitor, potent and selective CAS# 1477949-42-0 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1477949-42-0 | SDF | Download SDF |
| PubChem ID | 72946782 | Appearance | Powder |
| Formula | C15H22N2O3 | M.Wt | 278.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CAY-10683 | ||
| Solubility | DMSO : ≥ 100 mg/mL (359.26 mM) *"≥" means soluble, but saturation unknown. | ||
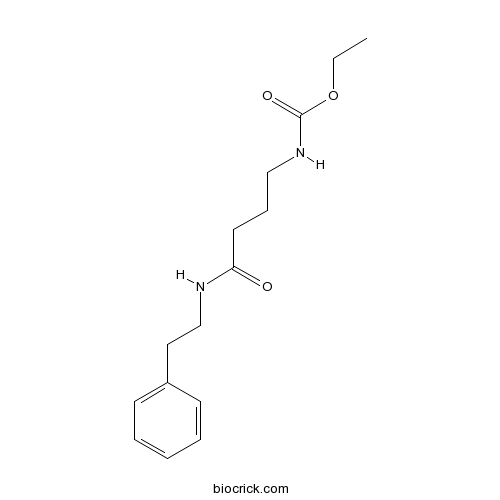
| Chemical Name | ethyl N-[4-oxo-4-(2-phenylethylamino)butyl]carbamate | ||
| SMILES | CCOC(=O)NCCCC(=O)NCCC1=CC=CC=C1 | ||
| Standard InChIKey | HTOYBIILVCHURC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H22N2O3/c1-2-20-15(19)17-11-6-9-14(18)16-12-10-13-7-4-3-5-8-13/h3-5,7-8H,2,6,9-12H2,1H3,(H,16,18)(H,17,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Santacruzamate A is a potent and selective histone deacetylase inhibitor. References: | |||||

Santacruzamate A (CAY10683) Dilution Calculator

Santacruzamate A (CAY10683) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5926 mL | 17.963 mL | 35.926 mL | 71.852 mL | 89.815 mL |
| 5 mM | 0.7185 mL | 3.5926 mL | 7.1852 mL | 14.3704 mL | 17.963 mL |
| 10 mM | 0.3593 mL | 1.7963 mL | 3.5926 mL | 7.1852 mL | 8.9815 mL |
| 50 mM | 0.0719 mL | 0.3593 mL | 0.7185 mL | 1.437 mL | 1.7963 mL |
| 100 mM | 0.0359 mL | 0.1796 mL | 0.3593 mL | 0.7185 mL | 0.8981 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Santacruzamate A (CAY10683) is a potent and selective inhibitor of histone deacetylase with IC50 values of 0.112 and 433 nM for HDAC2 and HDAC6, respectively [1].
Histone deacetylases (HDACs) are a series of enzymes that remove acetyl groups from an ε-N-acetyl lysine amino acid on a histone and make the histones to wrap the DNA more tightly, which prevent transcription.
Santacruzamate A (CAY10683) is a potent and selective HDAC inhibitor. Natural and synthetic santacruzamate A inhibited HDAC2 with IC50 values of 119 and 112 pM respectively and inhibited HDAC6 with IC50 values of 434 and 433 nM, respectively. Santacruzamate A was 700-fold more potent than SAHA for HDAC2. However, santacruzamate A inhibited HDAC4 with IC50 values of >1 µM. In HCT-116 colon carcinoma cells, natural and synthetic santacruzamate A inhibited cell growth with GI50 values of 29.4 and 28.3 µM, respectively. In HuT-78 cutaneous T-cell lymphoma cells, both inhibited cell growth with GI50 values of 1.4 and 1.3 µM, respectively [1].
Reference:
[1]. Pavlik CM, Wong CY, Ononye S, et al. Santacruzamate A, a potent and selective histone deacetylase inhibitor from the Panamanian marine cyanobacterium cf. Symploca sp. J Nat Prod, 2013, 76(11): 2026-2033.
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- 6-O-(3'',4''-Dimethoxycinnamoyl)catalpol
Catalog No.:BCN1655
CAS No.:147714-71-4
- 8-(3-Chlorostyryl)caffeine
Catalog No.:BCC7640
CAS No.:147700-11-6
- WIN 18446
Catalog No.:BCC6273
CAS No.:1477-57-2
- 2-Amino-4-methylbenzothiazole
Catalog No.:BCC8533
CAS No.:1477-42-5
- ZM 226600
Catalog No.:BCC6831
CAS No.:147695-92-9
- Magnolianin
Catalog No.:BCN3985
CAS No.:147663-91-0
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21(Waf1/Cip1) and p19(INK4d) upregulation in hepatocellular carcinoma.[Pubmed:29484736]
Cell Prolif. 2018 Jun;51(3):e12447.
OBJECTIVES: Histone deacetylases (HDACs) are commonly dysregulated in cancer and represent promising therapeutic targets. However, global HDAC inhibitors have shown limited efficacy in the treatment of solid tumours, including hepatocellular carcinoma (HCC). In this study, we investigated the therapeutic effect of selectively inhibiting HDAC1 and 2 in HCC. METHODS: HDAC1 inhibitor Tacedinaline (CI994), HDAC2 inhibitor Santacruzamate A (CAY10683), HDAC1/2 common inhibitor Romidepsin (FK228) and global HDAC inhibitor Vorinostat (SAHA) were used to treat HCC cells. Cell cycle, apoptosis and the protein levels of CDKs and CDKNs were performed to evaluate HCC cell growth. Inhibition of HDAC1/2 by RNAi was further investigated. RESULTS: Combined inhibition of HDAC1/2 led to HCC cell morphology changes, growth inhibition, cell cycle blockage and apoptosis in vitro and suppressed the growth of subcutaneous HCC xenograft tumours in vivo. p21(Waf1/Cip1) and p19(INK)(4d) , which play roles in cell cycle blockage and apoptosis induction, were upregulated. Inhibition of HDAC1/2 by siRNA further demonstrated that HDAC1 and 2 cooperate in blocking the cell cycle and inducing apoptosis via p19(INK)(4d) and p21(Waf1/Cip1) upregulation. Finally, H3K18, H3K56 and H4K12 in the p19(INK)(4d) and p21(Waf1/Cip1) promoter regions were found to be targets of HDAC1/2. CONCLUSIONS: Pharmacological or transcriptional inhibition of HDAC1/2 increases p19(INK)(4d) and p21(Waf1/Cip1) expression, decreases CDK expression and arrests HCC growth. These results indicated a potential pharmacological mechanism of selective HDAC1/2 inhibitors in HCC therapy.


