ThiabendazoleFungicide CAS# 148-79-8 |
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Necrostatin 2 racemate
Catalog No.:BCC2077
CAS No.:852391-15-2
- Necrostatin 2
Catalog No.:BCC1793
CAS No.:852391-19-6
- Necrostatin 2 S enantiomer
Catalog No.:BCC2078
CAS No.:852391-20-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148-79-8 | SDF | Download SDF |
| PubChem ID | 5430 | Appearance | Powder |
| Formula | C10H7N3S | M.Wt | 201.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2-(4-Thiazolyl)benzimidazole | ||
| Solubility | DMSO : 50 mg/mL (248.45 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
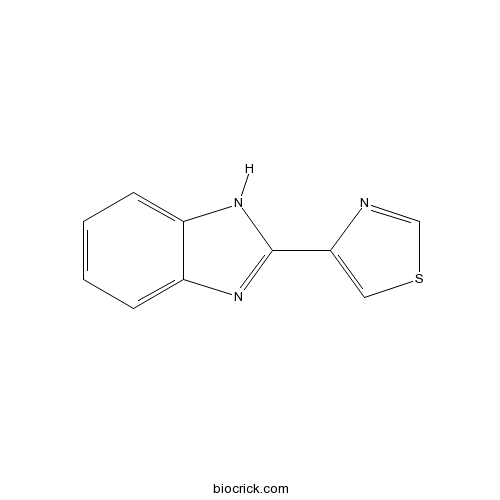
| Chemical Name | 4-(1H-benzimidazol-2-yl)-1,3-thiazole | ||
| SMILES | C1=CC=C2C(=C1)NC(=N2)C3=CSC=N3 | ||
| Standard InChIKey | WJCNZQLZVWNLKY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Thiabendazole inhibites the mitochondrial helminth-specific enzyme, fumarate reductase, with anthelminthic property.
Target: Fumarate Reductase
Tiabendazole serves to block angiogenesis in both frog embryos and human cells. It has also been shown to serve as a vascular disrupting agent to reduce newly established blood vessels. Tiabendazole has been shown to effectively do this in certain cancer cells. Thiabendazole works by inhibition of the mitochondrial, helminth-specific enzyme, fumarate reductase, with possible interaction with endogenous quinone [1].
Thiabendazole inhibited B16F10 proliferation in vitro in a dose- and time-dependent manner with an IC50 of 532.4 +/- 32.6, 322.9 +/- 28.9, 238.5 +/- 19.8 microM at 24, 48, and 72 h, respectively. Moreover, thiabendazole inhibited the angiogenesis and the migration of B16F10 cells in vitro. Furthermore, thiabendazole restrained transcription and translation of the VEGF gene in B16F10 in vitro, and the apoptotic percentage of B16F10 cells was increased after exposure to thiabendazole [2]. References: | |||||

Thiabendazole Dilution Calculator

Thiabendazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9689 mL | 24.8447 mL | 49.6894 mL | 99.3789 mL | 124.2236 mL |
| 5 mM | 0.9938 mL | 4.9689 mL | 9.9379 mL | 19.8758 mL | 24.8447 mL |
| 10 mM | 0.4969 mL | 2.4845 mL | 4.9689 mL | 9.9379 mL | 12.4224 mL |
| 50 mM | 0.0994 mL | 0.4969 mL | 0.9938 mL | 1.9876 mL | 2.4845 mL |
| 100 mM | 0.0497 mL | 0.2484 mL | 0.4969 mL | 0.9938 mL | 1.2422 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Thiabendazole is a benzimidazole compound that acts as a fungicide and is reported to have aneugenic effects. Rat hepatocyte studies suggest that Thiabendazole mediates the induction of CYP1A1 and anuran Xenopus laevis studies demonstrate its ability to elevate the mRNA expression of hsp70 and IL-1β. Other studies indicate that Thiabendazole acts as a time dependent inhibitor of CYP1A2.
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
Isolation of a bacterial consortium able to degrade the fungicide thiabendazole: the key role of a Sphingomonas phylotype.[Pubmed:28155070]
Appl Microbiol Biotechnol. 2017 May;101(9):3881-3893.
Thiabendazole (TBZ) is a fungicide used in fruit-packaging plants. Its application leads to the production of wastewaters requiring detoxification. In the absence of efficient treatment methods, biological depuration of these effluents could be a viable alternative. However, nothing is known regarding the microbial degradation of the recalcitrant and toxic to aquatics TBZ. We report the isolation, via enrichment cultures from a polluted soil, of the first bacterial consortium able to rapidly degrade TBZ and use it as a carbon source. Repeated efforts using various culture-dependent approaches failed to isolate TBZ-degrading bacteria in axenic cultures. Denaturating gradient gel electrophoresis (DGGE) and cloning showed that the consortium was composed of alpha-, beta- and gamma-Proteobacteria. Culture-independent methods including antibiotics-driven selection with DNA/RNA-DGGE, q-PCR and stable isotope probing (SIP)-DGGE identified a Sphingomonas phylotype (B13) as the key degrading member. Cross-feeding studies with structurally related chemicals showed that ring substituents of the benzimidazole moiety (thiazole or furan rings) favoured the cleavage of the imidazole moiety. LC-MS/MS analysis verified that TBZ degradation proceeds via cleavage of the imidazole moiety releasing thiazole-4-carboxamidine, which was not further transformed, and the benzoyl moiety, possibly as catechol, which was eventually consumed by the bacterial consortium as suggested by SIP-DGGE.
Enhanced fluorescence of terbium with thiabendazole and application in determining trace amounts of terbium and thiabendazole.[Pubmed:27837868]
Talanta. 2017 Jan 1;162:540-546.
In this paper, a simple, rapid and sensitive fluorescence method based on the formation of terbium (Tb(3+)) complex has been developed for the rapid detection of Tb(3+) in water. The fluorescence sensor has been studied by using terbium complexed with Thiabendazole (TBZ) while acetonitrile (MeCN) as solvent. The complex was made up of TBZ as small-molecule ligand and Tb(3+) as central ion. Fluorescence spectroscopy and UV spectroscopy together were used to study the behavior of the complexation of TBZ-Tb in this medium. Enhancement fluorescence was observed due to the efficient energy transfer process from TBZ to Tb(3+). And the affecting factors of the enhancement fluorescence were also studied in detail. Under optimal conditions, a linear relationship was obtained between the enhanced fluorescence intensity and the concentration of Tb(3+) in a range of 5.0x10(-6)M-3.0x10(-5)M. The sensor was used for the detection of Tb(3+) in river water samples, and the result was satisfactory. In the meantime, a linear relationship was also obtained between the enhanced fluorescence intensity and the concentration of TBZ in a range of 8.0x10(-6)M-4.0x10(-5)M. Moreover, the method was successfully extended for the detection of TBZ in juice samples.
Simultaneous Electrochemical Detection of Benzimidazole Fungicides Carbendazim and Thiabendazole Using a Novel Nanohybrid Material-Modified Electrode.[Pubmed:28068083]
J Agric Food Chem. 2017 Feb 1;65(4):727-736.
In this work, a novel ZnFe2O4/SWCNTs nanohybrid was successfully synthesized as electrode material and applied to the simultaneous quantitative determination of carbendazim (CBZ) and Thiabendazole (TBZ). The electrochemical behaviors of CBZ and TBZ on the ZnFe2O4/SWCNTs/GCE were investigated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The electrochemical active area of modified electrode was calculated, which is nearly 5.5 times that of the bare electrode. The influence of various factors such as accumulation time, pH and scan rates, type of surfactant, and the electrochemical reaction mechanism was studied. The results showed that the reaction of CBZ/TBZ was controlled by adsorption/diffusion and was a quasi-reversible/an irreversible process at the ZnFe2O4/SWCNTs/GCE. In the pH 7.0 phosphate-buffered saline (PBS) containing 10.0 mug/mL CTAB, the electrochemical responses of CBZ and TBZ were separately investigated and were linearly dependent on their concentrations ranging from 0.5 to 100.0 muM, with relatively low detection limits of 0.09 and 0.05 muM, respectively. The concentration range for the simultaneous determination of CBZ and TBZ was 1.0-100.0 muM. Furthermore, with satisfactory results, the proposed electrochemical sensor was successfully applied to the determination of CBZ and TBZ in the real samples.
Electromembrane extraction and preconcentration of carbendazim and thiabendazole in water samples before capillary electrophoresis analysis.[Pubmed:28165201]
J Sep Sci. 2017 Apr;40(7):1532-1539.
Electromembrane extraction using a polypropylene hollow fiber impregnated with 1-ethyl-2-nitrobenzene was evaluated for the extraction and preconcentration of the fungicides Thiabendazole and carbendazim from water samples before capillary electrophoresis analysis. The composition of the supported liquid membrane, the HCl concentration in the acceptor solution, and the stirring rate (of the donor solution) were optimized using the one-variable-at-a-time method. In contrast, a face-centered central composition design was used for optimization of voltage, extraction time, and concentration of HCl in the donor solution. After optimization, electromembrane extraction provided enrichment factors of 50 and 26 for Thiabendazole and carbendazim that allowed us to achieve limits of detection of 1.1 and 2.3 mug/L, respectively. Repeatability (intraday precision) expressed as the relative standard deviation varied from 2.5 to 2.8%, while the interday precision ranged from 3.1 to 3.3%. The proposed method was applied for analysis of samples of tap and river water, and acceptable precision and accuracy were attained.


