Ac-Lys(Fmoc)-OHCAS# 148101-51-3 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148101-51-3 | SDF | Download SDF |
| PubChem ID | 74788276 | Appearance | Powder |
| Formula | C23H26N2O5 | M.Wt | 410.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
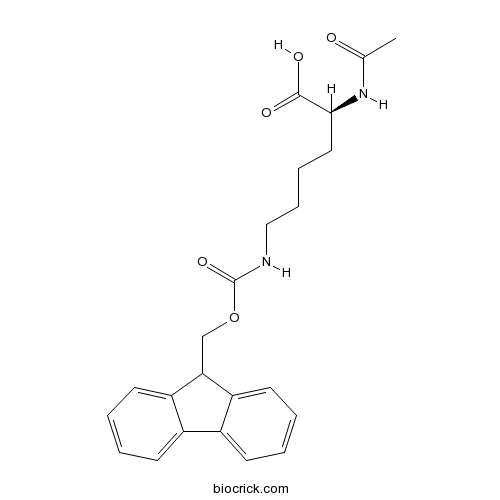
| Chemical Name | (2S)-2-acetamido-6-(9H-fluoren-9-ylmethoxycarbonylamino)hexanoic acid | ||
| SMILES | CC(=O)NC(CCCCNC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13)C(=O)O | ||
| Standard InChIKey | HPXGLFPXINBZFY-NRFANRHFSA-N | ||
| Standard InChI | InChI=1S/C23H26N2O5/c1-15(26)25-21(22(27)28)12-6-7-13-24-23(29)30-14-20-18-10-4-2-8-16(18)17-9-3-5-11-19(17)20/h2-5,8-11,20-21H,6-7,12-14H2,1H3,(H,24,29)(H,25,26)(H,27,28)/t21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ac-Lys(Fmoc)-OH Dilution Calculator

Ac-Lys(Fmoc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4363 mL | 12.1815 mL | 24.3629 mL | 48.7258 mL | 60.9073 mL |
| 5 mM | 0.4873 mL | 2.4363 mL | 4.8726 mL | 9.7452 mL | 12.1815 mL |
| 10 mM | 0.2436 mL | 1.2181 mL | 2.4363 mL | 4.8726 mL | 6.0907 mL |
| 50 mM | 0.0487 mL | 0.2436 mL | 0.4873 mL | 0.9745 mL | 1.2181 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4873 mL | 0.6091 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ac-Lys(Fmoc)-OH
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
- JMV 390-1
Catalog No.:BCC5922
CAS No.:148473-36-3
- MNS
Catalog No.:BCC3943
CAS No.:1485-00-3
- GRK2i
Catalog No.:BCC6048
CAS No.:148505-03-7
Synthesis and conformational analysis of a cyclic peptide obtained via i to i+4 intramolecular side-chain to side-chain azide-alkyne 1,3-dipolar cycloaddition.[Pubmed:18489158]
J Org Chem. 2008 Aug 1;73(15):5663-74.
Intramolecular side-chain to side-chain cyclization is an established approach to achieve stabilization of specific conformations and a recognized strategy to improve resistance toward proteolytic degradation. To this end, cyclizations, which are bioisosteric to the lactam-type side-chain to side-chain modification and do not require orthogonal protection schemes, are of great interest. Herein, we report the employment of Cu(I)-catalyzed 1,3-dipolar cycloaddition of side chains modified with azido and alkynyl functions and explore alternative synthetic routes to efficiently generate 1,4-disubstituted [1,2,3]triazolyl-containing cyclopeptides. The solid-phase assembly of the linear precursor including epsilon-azido norleucine and the propargylglycine (Pra) in positions i and i+4, respectively, was accomplished by either subjecting the resin-bound peptide to selective on-resin diazo transformation of a Lys into the Nle(epsilon-N3) or the incorporation of Fmoc-Nle(epsilon-N3)-OH during the stepwise build-up of the resin-bound peptide 1b. Solution-phase Cu(I)-catalyzed 1,3-dipolar cycloaddition converts the linear precursor Ac-Lys-Gly-Nle(epsilon-N3)-Ser-Ile-Gln-Pra-Leu-Arg-NH2 (2) into the 1,4-disubstituted [1,2,3]triazolyl-containing cyclopeptide [Ac-Lys-Gly-Xaa(&(1))-Ser-Ile-Gln-Yaa(&(2))-Leu-Arg-NH2][(&(1)(CH2)4-1,4-[1,2,3]t riazolyl-CH2&(2))] (3). The conformational preferences of the model cyclopeptide 3 (III), which is derived from the sequence of a highly helical and potent i to i+4 side-chain to side-chain lactam-containing antagonist of parathyroid hormone-related peptide (PTHrP), are compared to the corresponding lactam analogue Ac[Lys(13)(&(1)),Asp(17)(&(2))]hPTHrP(11-19)NH2 (II). CD and NMR studies of 3 and II in water/hexafluoroacetone (HFA) (50:50, v/v) revealed a high prevalence of turn-helical structures involving in particular the cyclic regions of the molecule. Despite a slight difference of the backbone arrangement, the side-chains of Ser, Gln, and Ile located at the i+1 to i+3 of the ring-forming sequences share the same spatial orientation. Both cyclopeptides differ regarding the location of the turn-helical segment, which in II involves noncyclized residues while in 3 it overlaps with residues involved in the cyclic structure. Therefore, the synthetic accessibility and conformational similarity of i to i+4 side-chain to side-chain cyclopeptide containing the 1,4-disubstituted [1,2,3]triazolyl moiety to the lactam-type one may result in similar bioactivities.


