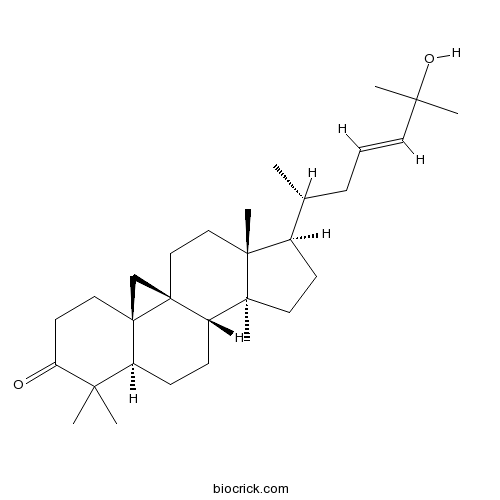
25-Hydroxycycloart-23-en-3-oneCAS# 148044-47-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148044-47-7 | SDF | Download SDF |
| PubChem ID | 44423578 | Appearance | Powder |
| Formula | C30H48O2 | M.Wt | 440.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(CC=CC(C)(C)O)C1CCC2(C1(CCC34C2CCC5C3(C4)CCC(=O)C5(C)C)C)C | ||
| Standard InChIKey | FQNGHHPZIYLPNI-DYWBVCMMSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Biotransformation of cycloartane-type triterpenes by the fungus Glomerella fusarioides.[Pubmed: 16643035]J Nat Prod. 2006 Apr;69(4):604-7.Biotransformation of three cycloartane-type triterpenes, cycloartenol (1), 24-methylenecycloartanol (2), and cycloartenone (3), by the fungus Glomerella fusarioides was studied.
|
| Structure Identification | Chem Biodivers. 2013 Sep;10(9):1613-22.Cycloartanes from the gum resin of Gardenia gummifera L.f.[Pubmed: 24078595]The gum resin exuding from the leaf buds of Gardenia gummifera was investigated. |

25-Hydroxycycloart-23-en-3-one Dilution Calculator

25-Hydroxycycloart-23-en-3-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2691 mL | 11.3456 mL | 22.6912 mL | 45.3823 mL | 56.7279 mL |
| 5 mM | 0.4538 mL | 2.2691 mL | 4.5382 mL | 9.0765 mL | 11.3456 mL |
| 10 mM | 0.2269 mL | 1.1346 mL | 2.2691 mL | 4.5382 mL | 5.6728 mL |
| 50 mM | 0.0454 mL | 0.2269 mL | 0.4538 mL | 0.9076 mL | 1.1346 mL |
| 100 mM | 0.0227 mL | 0.1135 mL | 0.2269 mL | 0.4538 mL | 0.5673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
Biotransformation of cycloartane-type triterpenes by the fungus Glomerella fusarioides.[Pubmed:16643035]
J Nat Prod. 2006 Apr;69(4):604-7.
Biotransformation of three cycloartane-type triterpenes, cycloartenol (1), 24-methylenecycloartanol (2), and cycloartenone (3), by the fungus Glomerella fusarioides was studied. Compound 1 was converted to 3, cycloart-25-ene-3beta,24-diol (4), and cycloartane-3beta,24,25-triol (5). Compound 2 was metabolized to cycloeucalenol (6) and two new compounds, 24-methylcycloartane-3beta,24,24(1)-triol (7) and 24(1)-methoxy-24-methylcycloartane-3beta,24-diol (8). Compound 3 was converted into two new metabolites, 4alpha,4beta,14alpha-trimethyl-9beta,19-cyclopregnane-3,20-dione (9) and 25-hydroxy-24-methoxycycloartan-3-one (14), and four known compounds, viz., cycloartane-3,24-dione (10), 24-hydroxycycloart-25-en-3-one (11), (23E)-25-Hydroxycycloart-23-en-3-one (12), and 24,25-dihydroxycycloartan-3-one (13). The structures of four new metabolites, 7, 8, 9, and 14, were established by spectroscopic methods.
Cycloartanes from the gum resin of Gardenia gummifera L.f.[Pubmed:24078595]
Chem Biodivers. 2013 Sep;10(9):1613-22.
The gum resin exuding from the leaf buds of Gardenia gummifera was investigated. Eight new cycloartane triterpenes, 1-6, 8, and 10, together with two known triterpenes, 25-Hydroxycycloart-23-en-3-one (7) and cycloartenone (9), were isolated and identified by extensive NMR spectroscopy. For cycloartenone (9), full NMR assignments are given as these data were not available in the literature. Eight compounds possess a C(3)=O group, two are 3,4-secocycloartanes bearing a free C(3)OOH group; in one of the cycloartanes, gummiferartane-9 (10), ring A occurs as a seven-membered lactone.


