(±)-Epibatidinedopamine receptor antagonist CAS# 148152-66-3 |
- Alvespimycin
Catalog No.:BCC1346
CAS No.:467214-20-6
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Retaspimycin
Catalog No.:BCC1889
CAS No.:857402-23-4
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- 17-AAG Hydrochloride
Catalog No.:BCC1297
CAS No.:911710-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148152-66-3 | SDF | Download SDF |
| PubChem ID | 21946437 | Appearance | Powder |
| Formula | C11H13ClN2 | M.Wt | 208.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water and to 100 mM in ethanol | ||
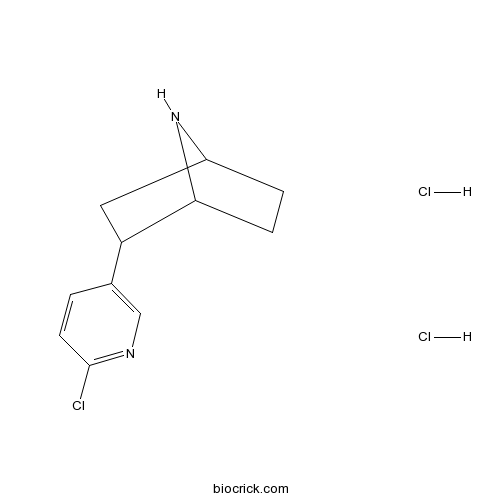
| Chemical Name | 3-(6-chloropyridin-3-yl)-7-azabicyclo[2.2.1]heptane;dihydrochloride | ||
| SMILES | C1CC2C(CC1N2)C3=CN=C(C=C3)Cl.Cl.Cl | ||
| Standard InChIKey | DGNNWUINALNROG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13ClN2.2ClH/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8;;/h1,4,6,8-10,14H,2-3,5H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity nicotinic agonist (Ki values are 0.02 and 233 nM for α4β2 and α7 nicotinic receptors respectively). Analgesic. |

(±)-Epibatidine Dilution Calculator

(±)-Epibatidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7918 mL | 23.959 mL | 47.918 mL | 95.8359 mL | 119.7949 mL |
| 5 mM | 0.9584 mL | 4.7918 mL | 9.5836 mL | 19.1672 mL | 23.959 mL |
| 10 mM | 0.4792 mL | 2.3959 mL | 4.7918 mL | 9.5836 mL | 11.9795 mL |
| 50 mM | 0.0958 mL | 0.4792 mL | 0.9584 mL | 1.9167 mL | 2.3959 mL |
| 100 mM | 0.0479 mL | 0.2396 mL | 0.4792 mL | 0.9584 mL | 1.1979 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(+)-AJ 76 hydrochloride is an antagonist of dopamine autoreceptor with pKi values of 6.95, 6.67, 6.37, 6.21 and 6.07 for hD3, hD4, hD2S, hD2L and rD2 receptors, respectively.
Dopamine receptor is a G protein-coupled receptor and mainly exists in the vertebrate central nervous system (CNS). Dopamine receptor is a receptor for dopamine and plays a critical role in memory, learning, pleasure, cognition, motivation and fine motor control.
(+)-AJ 76 hydrochloride is a dopamine receptor antagonist. In rats, AJ76 stimulated locomotor activity and increased the levels of 3,4-dihydroxyphenylacetic acid (DOPAC) and HVA in brain, which were dopamine metabolites [1]. In rats injected with cocaine, (+)-A J76 increased the locomotor stimulation during the first 30 min. However, (+)-AJ76 inhibited the later more intense locomotor stimulation and cocaine-induced stereotypies [2]. In vivo, (+)-AJ76 induced dopamine release mainly through interaction with dopamine receptors in the terminal regions of the A9 and A10 dopaminergic fibers. However, (+)-AJ76 increased the level of DOPAC via the somatodendritic autoreceptors [3].
References:
[1]. Kullingsjö H, Carlsson A, Svensson K. Effects of repeated administration of the preferential dopamine autoreceptor antagonist, (+)-AJ76, on locomotor activity and brain DA metabolism in the rat. Eur J Pharmacol, 1991, 205(3): 241-246.
[2]. Piercey MF, Lum JT, Hoffmann WE, et al. Antagonism of cocaine's pharmacological effects by the stimulant dopaminergic antagonists, (+)-AJ76 and (+)-UH232. Brain Res, 1992; 588(2): 217-222.
[3]. Waters N, Hansson L, Löfberg L, et al. Intracerebral infusion of (+)-AJ76 and (+)-UH232: effects on dopamine release and metabolism in vivo. Eur J Pharmacol, 1994, 251(2-3): 181-190.
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
- JMV 390-1
Catalog No.:BCC5922
CAS No.:148473-36-3
- MNS
Catalog No.:BCC3943
CAS No.:1485-00-3
- GRK2i
Catalog No.:BCC6048
CAS No.:148505-03-7
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
The binding orientation of epibatidine at alpha7 nACh receptors.[Pubmed:28089847]
Neuropharmacology. 2017 Apr;116:421-428.
Epibatidine is an alkaloid toxin that binds with high affinity to nicotinic and muscarinic acetylcholine receptors, and has been extensively used as a research tool. To examine binding interactions at the nicotinic receptor, it has been co-crystallised with the structural homologue acetylcholine binding protein (AChBP; PDB ID 2BYQ), and with an AChBP chimaera (3SQ6) that shares 64% sequence identity with the alpha7 nACh receptor. However, the binding orientations revealed by AChBP co-crystal structures may not precisely represent their receptor homologues and experimental evidence is needed to verify the ligand poses. Here we identify potential binding site interactions between epibatidine and AChBP residues, and substitute equivalent positions in the alpha7 nACh receptor. The effects of these are probed by [(3)H]epibatidine binding following the expression alpha7 nACh receptor cysteine mutants in HEK 293 cells. Of the sixteen mutants created, the affinity of epibatidine was unaffected by the substitutions Q55C, L106C, L116C, T146C, D160C and S162C, reduced by C186A and C187A, increased by Q114C and S144C, and abolished by W53C, Y91C, N104C, W145C, Y184C and Y191C. These results are consistent with the predicted orientations in AChBP and suggest that epibatidine is likely to occupy a similar location at alpha7 nACh receptors. We speculate that steric constraints placed upon the C-5 position of the pyridine ring in 3SQ6 may account for the relatively poor affinities of epibatidine derivatives that are substituted at this position.
Discriminative stimulus and hypothermic effects of some derivatives of the nAChR agonist epibatidine in mice.[Pubmed:24800895]
Psychopharmacology (Berl). 2014 Dec;231(23):4455-66.
RATIONALE: Receptor mechanisms underlying the in vivo effects of nicotinic acetylcholine receptor (nAChR) drugs need to be determined to better understand possible differences in therapeutic potential. OBJECTIVE: This study compared the effects of agonists that are reported either to differ in intrinsic activity (i.e., efficacy) at alpha4beta2 nAChR in vitro or to have in vivo effects consistent with differences in efficacy. The drugs included nicotine, varenicline, cytisine, epibatidine, and three novel epibatidine derivatives: 2'-fluoro-3'-(4-nitrophenyl)deschloroepibatidine (RTI-7527-102), 2'-fluorodeschloroepibatidine (RTI-7527-36), and 3'-(3''-dimethylaminophenyl)-epibatidine (RTI-7527-76). METHODS: Mice discriminated nicotine base (1 mg/kg base) from saline; other mice were used to measure rectal temperature. RESULTS: In the nicotine discrimination assay, the maximum percentage of nicotine-appropriate responding varied: 92 % for nicotine, 84 % for epibatidine, 77 % for RTI-7527-36, and 71 % for varenicline and significantly less for RTI-7527-76 (58 %), RTI-7527-102 (46 %), and cytisine (33 %). Each drug markedly decreased rectal temperature by as much as 12 masculineC. The rank-order potency in the discrimination and hypothermia assays was epibatidine > RTI-7527-36 > nicotine > RTI-7527-102 > varenicline = cytisine = RTI-7527-76. The nAChR antagonist mecamylamine (3.2 mg/kg) antagonized the discriminative stimulus effects of epibatidine and RTI-7527-102, as well as the hypothermic effects of every drug except cytisine. The beta2-subunit selective nAChR antagonist dihydro-beta-erythroidine (DHbetaE; up to 10 mg/kg) antagonized hypothermic effects but less effectively so than mecamylamine. CONCLUSIONS: The marked hypothermic effects of all drugs except cytisine are due in part to agonism at nAChR containing beta2-subunits. Differential substitution for the nicotine discriminative stimulus is consistent with differences in alpha4beta2 nAChR efficacy; however, collectively the current results suggest that multiple nAChR receptor subtypes mediate the effects of the agonists.
Possible inhibitory role of endogenous 2-arachidonoylglycerol as an endocannabinoid in (+/-)-epibatidine-induced activation of central adrenomedullary outflow in the rat.[Pubmed:25882827]
Neuropharmacology. 2015 Aug;95:278-89.
We previously reported that intracerebroventricularly (i.c.v.) administered (+/-)-epibatidine (1, 5 or 10 nmol/animal), a nicotinic acetylcholine receptor agonist, dose-dependently induced secretion of noradrenaline and adrenaline (catecholamines) from the rat adrenal medulla by brain diacylglycerol lipase- (DGL), monoacylglycerol lipase- (MGL) and cyclooxygenase-mediated mechanisms. Diacylglycerol is hydrolyzed by DGL into 2-arachidonoylglycerol (2-AG), which is further hydrolyzed by MGL to arachidonic acid (AA), a cyclooxygenase substrate. These findings suggest that brain 2-AG-derived AA is involved in the (+/-)-epibatidine-induced response. This AA precursor 2-AG is also a major brain endocannabinoid, which inhibits synaptic transmission through presynaptic cannabinoid CB1 receptors. Released 2-AG into the synaptic cleft is rapidly inactivated by cellular uptake. Here, we examined a role of brain 2-AG as an endocannabinoid in the (+/-)-epibatidine-induced activation of central adrenomedullary outflow using anesthetized male Wistar rats. In central presence of AM251 (CB1 antagonist) (90 and 180 nmol/animal, i.c.v.), (+/-)-epibatidine elevated plasma catecholamines even at an ineffective dose (1 nmol/animal, i.c.v.). Central pretreatment with ACEA (CB1 agonist) (0.7 and 1.4 mumol/animal, i.c.v.), 2-AG ether (stable 2-AG analog for MGL) (0.5 and 1.0 mumol/animal, i.c.v.) or AM404 (endocannabinoid uptake inhibitor) (80 and 250 nmol/animal, i.c.v.) significantly reduced an effective dose of (+/-)-epibatidine- (5 nmol/animal, i.c.v.) induced elevation of plasma catecholamines, and AM251 (90 and 180 nmol/animal, i.c.v.) centrally abolished the reduction induced by 2-AG ether (1.0 mumol/animal, i.c.v.) or AM404 (250 nmol/animal, i.c.v.). Immunohistochemical studies demonstrated that (+/-)-epibatidine (10 nmol/animal, i.c.v.) activated DGLalpha-positive spinally projecting neurons in the hypothalamic paraventricular nucleus, a control center of central adrenomedullary system. These results suggest a possibility that a brain endocannabinoid, probably 2-AG, plays an inhibitory role in (+/-)-epibatidine-induced activation of central adrenomedullary outflow through brain CB1 receptors in the rat.
Epibatidine blocks eye-specific segregation in ferret dorsal lateral geniculate nucleus during stage III retinal waves.[Pubmed:25794280]
PLoS One. 2015 Mar 20;10(3):e0118783.
The segregation and maintenance of eye-specific inputs in the dorsal lateral geniculate nucleus (dLGN) during early postnatal development requires the patterned spontaneous activity of retinal waves. In contrast to the development of the mouse, ferret eye-specific segregation is not complete at the start of stage III glutamatergic retinal waves, and the remaining overlap is limited to the C/C1 lamina of the dLGN. To investigate the role of patterned spontaneous activity in this late segregation, we disrupted retinal waves pharmacologically for 5 day windows from postnatal day (P) 10 to P25. Multi-electrode array recordings of the retina in vitro reveal that the cholinergic agonist epibatidine disrupts correlated retinal activity during stage III waves. Epibatidine also prevents the segregation of eye-specific inputs in vivo during that period. Our results reveal a novel role for cholinergic influence on stage III retinal waves as an instructive signal for the continued segregation of eye-specific inputs in the ferret dLGN.
UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes.[Pubmed:10751429]
J Neurosci. 2000 Apr 15;20(8):2783-91.
Presynaptic nicotinic acetylcholine receptors (nAChRs) on striatal synaptosomes stimulate dopamine release. Partial inhibition by the alpha3beta2-selective alpha-conotoxin-MII indicates heterogeneity of presynaptic nAChRs on dopamine terminals. We have used this alpha-conotoxin and UB-165, a novel hybrid of epibatidine and anatoxin-a, to address the hypothesis that the alpha-conotoxin-MII-insensitive subtype is composed of alpha4 and beta2 subunits. UB-165 shows intermediate potency, compared with the parent molecules, at alpha4beta2* and alpha3-containing binding sites, and resembles epibatidine in its high discrimination of these sites over alpha7-type and muscle binding sites. (+/-)-Epibatidine, (+/-)-anatoxin-a, and (+/-)-UB-165 stimulated [(3)H]-dopamine release from striatal synaptosomes with EC(50) values of 2.4, 134, and 88 nM, and relative efficacies of 1:0.4:0.2, respectively. alpha-Conotoxin-MII inhibited release evoked by these agonists by 48, 56, and 88%, respectively, suggesting that (+/-)-UB-165 is a very poor agonist at the alpha-conotoxin-MII-insensitive nAChR subtype. In assays of (86)Rb(+) efflux from thalamic synaptosomes, a model of an alpha4beta2* nAChR response, (+/-)-UB-165 was a very weak partial agonist; the low efficacy of (+/-)-UB-165 at alpha4beta2 nAChR was confirmed in Xenopus oocytes expressing various combinations of human nAChR subunits. In contrast, (+/-)-UB-165 and (+/-)-anatoxin-a were similarly efficacious and similarly sensitive to alpha-conotoxin-MII in increasing intracellular Ca(2+) in SH-SY5Y cells, a functional assay for native alpha3-containing nAChR. These data support the involvement of alpha4beta2* nAChR in the presynaptic modulation of striatal dopamine release and illustrate the utility of exploiting a novel partial agonist, together with a selective antagonist, to dissect the functional roles of nAChR subtypes in the brain.
Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain.[Pubmed:9536034]
J Pharmacol Exp Ther. 1998 Apr;285(1):377-86.
The binding of [3H]epibatidine, an alkaloid isolated from the skin of an Ecuadorean tree frog, was measured both in brain regions dissected from mouse brain and in tissue sections. Binding to each of 12 brain areas was saturable, but apparently monophasic; no indication of multiple binding sites was obtained. However, inhibition of epibatidine binding by nicotine, acetylcholine, methylcarbachol and cytisine in olfactory bulbs revealed a biphasic pattern consistent with the presence of two sites differentially sensitive to inhibition by these nicotinic agonists. Cytisine displayed the greatest difference in inhibitory potency between the two apparent sites. Subsequent analysis of the inhibition of epibatidine binding by cytisine in membranes prepared from 12 brain areas also suggested the presence of two sites in each brain region. The estimated potency of cytisine at each site was similar in each brain region. However, the proportion of [3H]epibatidine binding sites that were more sensitive to inhibition by cytisine and those sites less sensitive to inhibition by this agonist varied markedly among the brain regions. Quantitative autoradiographic analyses of mouse brain revealed pattern of [3H]epibatidine binding sites less sensitive to inhibition by cytisine that differed markedly from the pattern obtained with [3H]nicotine. Among brain regions demonstrating substantial sites less sensitive to cytisine inhibition were the accessory olfactory nucleus, medial habenula, interpeduncular nucleus, fasciculus retroflexus, superior colliculus, inferior colliculus and the pineal gland. The results indicate that epibatidine binds to at least two distinct nicotinic sites in mouse brain that may represent different nicotinic receptor subtypes, one of which appears to be identical to that measured by the binding of other agonists such as nicotine or cytisine.
Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors.[Pubmed:7476906]
Mol Pharmacol. 1995 Oct;48(4):774-82.
Pharmacological properties of the (+)- and (-)-isomers of synthetic epibatidine, exo-2-(6-chloro-3-pyridyl)-7-azabicyclo-[2.2.1]heptane, were compared with nicotine and acetylcholine on several subtypes of chicken and human nicotinic acetylcholine receptors (AChRs). Both isomers of epibatidine behaved as extremely potent full agonists on chicken (alpha 3 beta 2, alpha 3 beta 4, alpha 4 beta 2, alpha 7, and alpha 8) and human (alpha 3 beta 2, alpha 3 beta 4, and alpha 7) neuronal AChRs expressed in Xenopus oocytes. Currents induced by epibatidine were effectively blocked by the nicotinic antagonists hexamethonium and mecamylamine. Apparent affinity was 100 to 1000-fold higher for epibatidine than for nicotine or acetylcholine. EC50 values ranged from 1 nM (for homomeric chicken alpha 8) to 2 microM (for homomeric chicken alpha 7). Epibatidine showed comparatively lower affinity for muscle-type AChRs from Torpedo and humans (EC50 values, 1.6 and 16 microM respectively). In binding assays, epibatidine was used on AChR subtypes immunoisolated from chicken brain and retina (alpha 4 beta 2, alpha 7, and alpha 8), the human neuronal cell line SH-SY5Y (alpha 3 and alpha 7), Torpedo electric organ (alpha 1 beta 1 gamma delta), or the human rhabdomyosarcoma cell line TE671 (alpha 1 beta 1 gamma delta). Both isomers of epibatidine exhibited extremely high affinity for all neuronal AChRs tested, with KI values ranging from 0.6 pM (human alpha 3 AChRs) to 0.6 microM (chicken alpha 7 AChRs). In contrast, epibatidine had lower affinity for Torpedo muscle-type AChRs (KI approximately 5 microM). Racemic [3H]epibatidine was an effective labeling reagent for human alpha 3 beta 2 AChRs, exhibiting a KD (0.14 nM) similar to the KI values observed for unlabeled (+)-epibatidine (0.23 nM) or (-)-epibatidine (0.16 nM).
Epibatidine, a potent analgetic and nicotinic agonist.[Pubmed:8183234]
Mol Pharmacol. 1994 Apr;45(4):563-9.
Synthetic (+)- and (-)-epibatidine (an alkaloid originally characterized from frog skin) have potent analgetic activity in mice, using the hot-plate assay. The natural (+)-enantiomer, with an ED50 of about 1.5 micrograms/kg upon intraperitoneal injection, is about 2-fold more potent than the (-)-enantiomer. The analgetic activity is blocked by the nicotinic antagonist mecamylamine. Both the (+)- and (-)-enantiomers have high affinity (Ki values of 0.045 and 0.058 nm, respectively) for nicotinic sites that bind [3H] nicotine in rat brain membranes. An analog of epibatidine with the chloro substituent of the pyridyl ring replaced with hydrogen has comparable affinity for nicotinic sites, whereas replacement with a methyl or iodo substituent lowers activity. Both (+)- and (-)-epibatidine have potent agonist activity at ganglionic-type nicotinic receptors in pheochromocytoma PC-12 cells, with EC50 values for stimulation of sodium influx of 72 and 111 nM, respectively. (-)-Epibatidine is about 5-fold less potent as an agonist at muscle-type central nicotinic receptors of medulloblastoma TE671 cells. It would appear that the analgetic activity of epibatidine is due to activity as a nicotinic agonist. The epibatidines have little or no activity at a variety of other central receptors, including opioid receptors, muscarinic receptors, adrenergic receptors, dopamine receptors, serotonin receptors, and gamma-aminobutyric acid receptors.


