CA-074 MeCathepsin B inhibitor CAS# 147859-80-1 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147859-80-1 | SDF | Download SDF |
| PubChem ID | 6610318 | Appearance | Powder |
| Formula | C19H31N3O6 | M.Wt | 397.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CA-074Me | ||
| Solubility | DMSO : ≥ 215 mg/mL (540.92 mM) H2O : 26.66 mg/mL (67.07 mM; Need warming) *"≥" means soluble, but saturation unknown. | ||
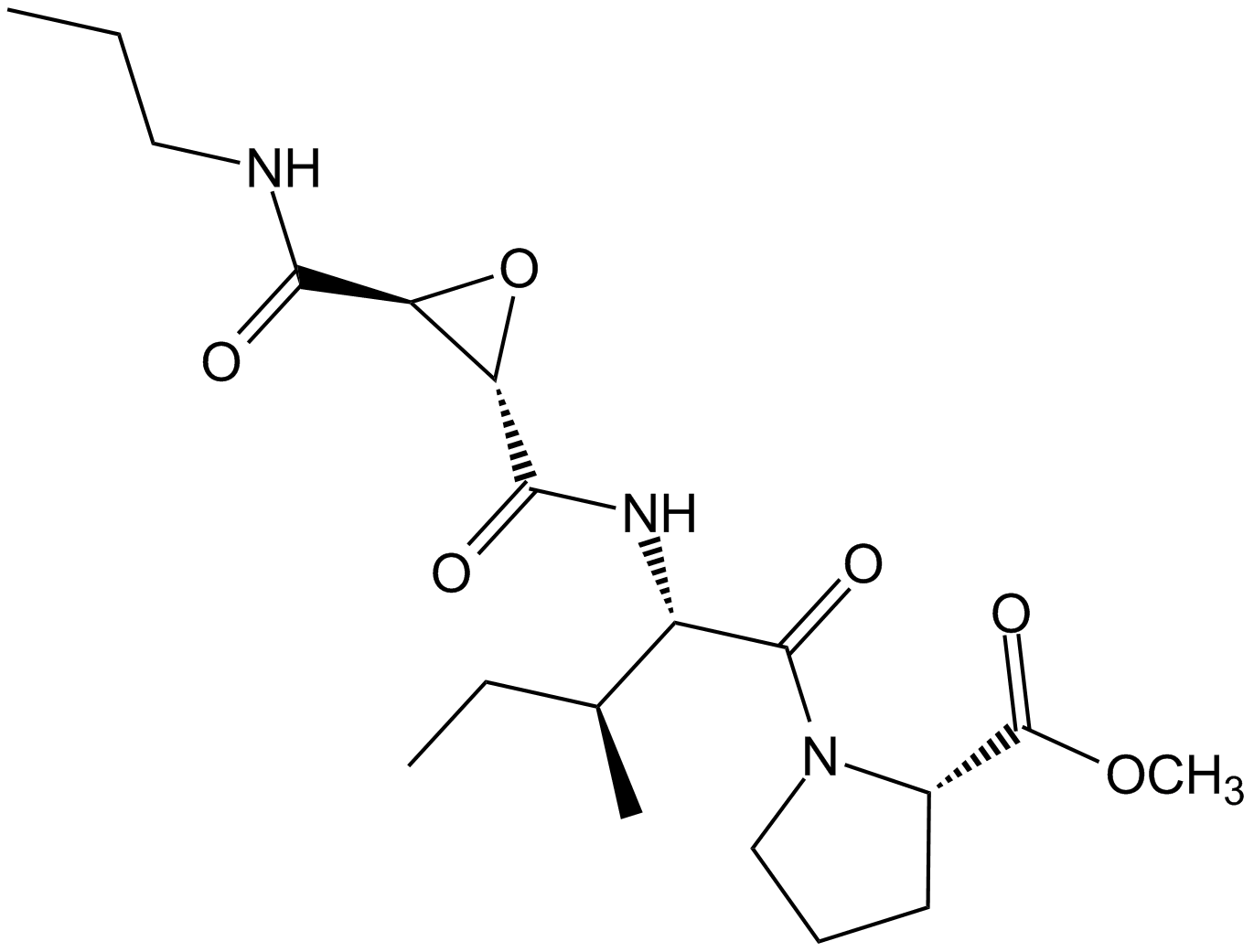
| Chemical Name | methyl (2S)-1-[(2S,3S)-3-methyl-2-[[(2S,3S)-3-(propylcarbamoyl)oxirane-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carboxylate | ||
| SMILES | CCCNC(=O)C1C(O1)C(=O)NC(C(C)CC)C(=O)N2CCCC2C(=O)OC | ||
| Standard InChIKey | XGWSRLSPWIEMLQ-YTFOTSKYSA-N | ||
| Standard InChI | InChI=1S/C19H31N3O6/c1-5-9-20-16(23)14-15(28-14)17(24)21-13(11(3)6-2)18(25)22-10-7-8-12(22)19(26)27-4/h11-15H,5-10H2,1-4H3,(H,20,23)(H,21,24)/t11-,12-,13-,14-,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CA-074 Me is a selective and cell-permeable inhibitor of cathepsin B. | |||||
| Targets | cathepsin B | |||||
| IC50 | 2.2 nM | |||||
| Cell experiment: [1] | |
| Cell lines | McNtcp.24 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 0.1 µM, 2 hours |
| Applications | Cells were incubated in medium alone or with 50 µM GCDC in the absence or presence of 0.1 µM CA-074 Me. Apoptosis was quantitated after 2 h of incubation. The cathepsin B inhibitor CA-074 Me reduced the GCDC-mediated increase in cathepsin B activity and apoptosis in McNtcp.24 cells. The result confirms that cathepsin B activity increases and contributes to bile salt–mediated apoptosis in primary rat hepatocytes. |
| Animal experiment: [2] | |
| Animal models | CatB+/+ mice |
| Dosage form | Intraperitoneal injection, 4 mg/100g |
| Application | Serum ALT levels after TNF-α-treatment were significantly reduced in catB+/+ mice pretreated with CA-074 Me compared to saline-injected controls. In contrast, liver architecture was preserved and only moderate damage was observed in catB+/+ mice pretreated with CA-074 Me. These results suggest that pharmacological inhibition of cat B may partially attenuate TNF-α-induced liver damage. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Faubion W A, Guicciardi M E, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. The Journal of clinical investigation, 1999, 103(1): 137-145. [2] Guicciardi M E, Miyoshi H, Bronk S F, et al. Cathepsin B knockout mice are resistant to tumor necrosis factor-α-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. The American journal of pathology, 2001, 159(6): 2045-2054. | |

CA-074 Me Dilution Calculator

CA-074 Me Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CA-074 Me is a membrane-permeable and selective inhibitor of cathepsin B with IC50 value of 36.3 nM [1, 2].
CA-074 Me is a methyl ester derivative of CA-074. In cultured human gingival fibroblasts, CA-074 Me exerted a 95% inhibition of cathepsin B and partial inhibition (54%) of the combined activities of cathepsins B and L. CA-074 Me was also found to inhibit cathespin L under reducing conditions. It inhibited the activity of purified human cathepsin L by more than 90% when the enzyme had been pre-incubated with 1.4 mM DTT or 4.2 mM GSH for 2 hours. Besides that, CA-074 Me completely inhibited cathepsin B in the presence of 1.4 mM DTT [2, 3].
References:
[1] Wu X, Zhang L, Gurley E, et al. Prevention of free fatty acid–induced hepatic lipotoxicity by 18β-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology, 2008, 47(6): 1905-1915.
[2] Steverding D. The cathepsin B-selective inhibitors CA-074 and CA-074Me inactivate cathepsin L under reducing conditions. Open Enzyme Inhibition Journal, 2011, 4: 11-16.
[3] Buttle D J, Murata M, Knight C G, et al. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Archives of biochemistry and biophysics, 1992, 299(2): 377-380.
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Niazimicin
Catalog No.:BCN7641
CAS No.:147821-49-6
- Siramesine
Catalog No.:BCC4304
CAS No.:147817-50-3
- Cefcapene pivoxil hydrochloride
Catalog No.:BCC8906
CAS No.:147816-24-8
- Santacruzamate A (CAY10683)
Catalog No.:BCC5488
CAS No.:1477949-42-0
- ω-Conotoxin MVIIC
Catalog No.:BCC5699
CAS No.:147794-23-8
- DCG IV
Catalog No.:BCC5691
CAS No.:147782-19-2
- L-161,982
Catalog No.:BCC7393
CAS No.:147776-06-5
- Repaglinide ethyl ester
Catalog No.:BCC9135
CAS No.:147770-06-7
- Fmoc-O-Phospho-Tyr-OH
Catalog No.:BCC3563
CAS No.:147762-53-6
- 3,4-Dimethoxybenzenepropanamine
Catalog No.:BCN1785
CAS No.:14773-42-3
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
Patulin Induces Autophagy-Dependent Apoptosis through Lysosomal-Mitochondrial Axis and Impaired Mitophagy in HepG2 Cells.[Pubmed:30392375]
J Agric Food Chem. 2018 Nov 21;66(46):12376-12384.
Patulin (PAT) is a compound produced by fungi including those of the Aspergillus, Penicillium, and Byssochlamys species. PAT has been linked with negative outcomes in certain microorganisms and animal species, but how it causes hepatotoxicity is poorly understood. In this study, we determined that, by treating HepG2 cells using PAT, these cells could be induced to rapidly undergo autophagy, and this was followed within 12 h of treatment by lysosomal membrane permeabilization (LMP) and cathepsin B release. We were able to block these outcomes if cells were treated with 3-methyladenine (3MA), an inhibitor of autophagy, prior to PAT treatment. Moreover, PAT-induced collapse of mitochondrial membrane potential (DeltaPsim) depended both on cathepsin B and autophagy. 3MA was further able to reduce the induction of apoptosis in response to PAT, suggesting that autophagy is a driving mechanism for this apoptotic induction. Inhibiting cathepsin B using CA-074 Me further reduced PAT-induced collapses of DeltaPsim, mitochondiral cytochrome c release, and apoptosis. We also found that extended treatment of HepG2 cells using PAT over a period of 24 h led to the impairment of mitophagy such that morphologically swollen mitochondria accumulated within cells, and PINK1 failed to colocalize with LC3. Together these data reveal that PAT treatment can promote the induction of apoptosis in HepG2 cells in a manner dependent upon autophagy that progresses via the lysosomal-mitochondrial axis. This study thereby affords new insights into the mechanisms by which PAT drives hepatotoxicity.
Lysosomal membrane permeabilization causes secretion of IL-1beta in human vascular smooth muscle cells.[Pubmed:30136196]
Inflamm Res. 2018 Oct;67(10):879-889.
OBJECTIVE: IL-1beta secretion by the inflammasome is strictly controlled and requires two sequential signals: a priming signal and an activating signal. Lysosomal membrane permeabilization (LMP) plays a critical role in the regulation of NLRP3 inflammasome, and generally acts as an activating signal. However, the role of LMP controlling NLRP3 inflammasome activation in human vascular smooth muscle cells (hVSMCs) is not well defined. METHODS: LMP was induced in hVSMCs by Leu-Leu-O-methyl ester. Cathepsin B was inhibited by CA-074 Me. Cytokine release, mRNA, and protein were quantified by enzyme-linked immunosorbent assay, quantitative PCR, and Western blot, respectively. NF-kappaB activity was analyzed by immunostaining of the NF-kappaB p65 nuclear translocation and using the dual-luciferase reporter assay system. RESULTS: LMP had both priming and activating roles, causing an upregulation of proIL-1beta and NLRP3 and the secretion of mature IL-1beta from unprimed hVSMCs. LMP activated the canonical NF-kappaB pathway. The priming effect of LMP was inhibited by CA-074 Me, indicating an upstream role of cathepsin B. CONCLUSIONS: These data support a novel role of LMP as a single stimulus for the secretion of IL-1beta from hVSMCs, implying the possibility that hVSMCs are an important initiator of the sterile inflammatory response caused by lysosomal disintegration.
Acrolein-induced autophagy-dependent apoptosis via activation of the lysosomal-mitochondrial pathway in EAhy926 cells.[Pubmed:29902662]
Toxicol In Vitro. 2018 Oct;52:146-153.
Acrolein, a highly reactive alpha,beta-unsaturated aldehyde, is a toxic component of cigarette smoke. As a lipid peroxidation biomarker, acrolein plays an important role in a wide variety of disease states, such as neurodegenerative, Alzheimer's disease, diabetes and atherosclerosis. Endothelial cell injury is one of the initiating factors of atherosclerosis, but the underlying molecular mechanisms remain unclear. Our study primarily focused on acrolein-induced autophagy-dependent apoptosis and the possible molecular mechanism. The results showed that treatment with acrolein increased the number of intracellular GFP-LC3 II punctuates and the expression of autophagosome biomarker LC3-II, with the low dose (25muM) or at the early stage of treatment (3h). Following treatment of EAhy926 cells with acrolein for 6h, lysosomal permeabilization changed, and cathepsin B (CB) was released. Additionally, acrolein induced the collapse of mitochondrial transmembrane potential, and cytochrome c was released. Furthermore, caspase-3 and caspase-9 activation showed that acrolein induced EAhy926 cell apoptosis. Autophagy inhibitor 3MA and CB inhibitor CA-074 Me (CA) attenuated acrolein-induced apoptosis. Collectively, our results suggested that acrolein-induced apoptosis is autophagy-dependent, occurring via injury to lysosomes and mitochondria. This study provides new mechanistic insight toward understanding the pathogenesis of acrolein-related disorders.


