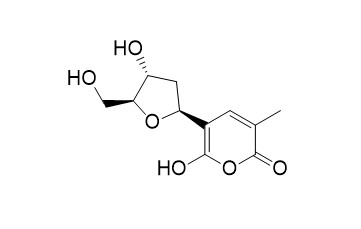
TetillapyroneCAS# 363136-43-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 363136-43-0 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C11H14O6 | M.Wt | 242.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tetillapyrone Dilution Calculator

Tetillapyrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1288 mL | 20.6441 mL | 41.2882 mL | 82.5764 mL | 103.2205 mL |
| 5 mM | 0.8258 mL | 4.1288 mL | 8.2576 mL | 16.5153 mL | 20.6441 mL |
| 10 mM | 0.4129 mL | 2.0644 mL | 4.1288 mL | 8.2576 mL | 10.322 mL |
| 50 mM | 0.0826 mL | 0.4129 mL | 0.8258 mL | 1.6515 mL | 2.0644 mL |
| 100 mM | 0.0413 mL | 0.2064 mL | 0.4129 mL | 0.8258 mL | 1.0322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lariciresinol 4'-O-glucoside
Catalog No.:BCN0774
CAS No.:107110-16-7
- 3'-Hydroxy-5,7,4'-trimethoxyflavone
Catalog No.:BCN0773
CAS No.:33554-52-8
- 4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No.:BCN0772
CAS No.:85644-03-7
- (-)-5'-Methoxyisolariciresinol 3alpha-O-beta-glucopyranoside
Catalog No.:BCN0771
CAS No.:143236-03-7
- Meliotocarpan D
Catalog No.:BCN0770
CAS No.:83013-81-4
- Tumulosic acid
Catalog No.:BCN0769
CAS No.:508-24-7
- 3'-Hydroxy-5,7,4',5'-Tetramethoxyflavone
Catalog No.:BCN0768
CAS No.:29976-51-0
- Platycodin J
Catalog No.:BCN0767
CAS No.:1325614-80-9
- Dipsacus saponin X
Catalog No.:BCN0759
CAS No.:146100-01-8
- 7-O-(4-beta-D-glucopyranosyloxy-3-methoxybenzoyl)secologanolic acid
Catalog No.:BCN0758
CAS No.:469899-55-6
- Eucomic acid
Catalog No.:BCN0757
CAS No.:42151-32-6
- ZP-amide C
Catalog No.:BCN0756
CAS No.:412316-38-2
- 4'-Hydroxy-5,7,3'-trimethoxyflavone
Catalog No.:BCN0776
CAS No.:1239-68-5
- (2S)-5,7,3',4'-tetramethoxyflavanone
Catalog No.:BCN0777
CAS No.:74628-43-6
- (Z)-3,11-dimethy-7-methylene-9,14-epoxy-1,6,10-dodecatrien-3-ol
Catalog No.:BCN0778
CAS No.:1392202-57-1
- Quercetin 3,7-diglucoside
Catalog No.:BCN0779
CAS No.:6892-74-6
- 6alpha-Hydroxydehydropachymic acid
Catalog No.:BCN0780
CAS No.:176390-67-3
- 3',4',5',5,7-Pentamethoxyflavanone
Catalog No.:BCN0781
CAS No.:479672-30-5
- Erythro-Guaiacylglycerol-beta-coniferyl aldehyde ether
Catalog No.:BCN0782
CAS No.:74474-55-8
- Naringin 6''-acetate
Catalog No.:BCN0783
CAS No.:139934-60-4
- Murracarpin
Catalog No.:BCN0784
CAS No.:120786-76-7
- Melitidin
Catalog No.:BCN0785
CAS No.:1162664-58-5
- threo-Guaiacylglycerol-beta-coniferyl aldehyde ether
Catalog No.:BCN0786
CAS No.:650600-33-2
- Citrusin C
Catalog No.:BCN0787
CAS No.:18604-50-7
Cytotoxic Compounds from the Saudi Red Sea Sponge Xestospongia testudinaria.[Pubmed:27128926]
Mar Drugs. 2016 Apr 26;14(5). pii: md14050082.
Bioassay-guided fractionation of the organic extract of the Red Sea sponge Xestospongia testudinaria led to the isolation of 13 compounds including two new sterol esters, xestosterol palmitate (2) and xestosterol ester of l6'-bromo-(7'E,11'E,l5'E)-hexadeca-7',11',l5'-triene-5',13'-diynoic acid (4), together with eleven known compounds: xestosterol (1), xestosterol ester of 18'-bromooctadeca-7'E,9'E-diene-7',15'-diynoic acid (3), and the brominated acetylenic fatty acid derivatives, (5E,11E,15E,19E)-20-bromoeicosa-5,11,15,19-tetraene-9,17-diynoic acid (5), 18,18-dibromo-(9E)-octadeca-9,17-diene-5,7-diynoic acid (6), 18-bromooctadeca-(9E,17E)-diene-7,15-diynoic acid (7), 18-bromooctadeca-(9E,13E,17E)-triene-7,15-diynoic acid (8), l6-bromo (7E,11E,l5E)hexadeca-7,11,l5-triene-5,13-diynoic acid (9), 2-methylmaleimide-5-oxime (10), maleimide-5-oxime (11), Tetillapyrone (12), and norTetillapyrone (13). The chemical structures of the isolated compounds were accomplished using one- and two-dimensional NMR, infrared and high-resolution electron impact mass spectroscopy (1D, 2D NMR, IR and HREIMS), and by comparison with the data of the known compounds. The total alcoholic and n-hexane extracts showed remarkable cytotoxic activity against human cervical cancer (HeLa), human hepatocellular carcinoma (HepG-2), and human medulloblastoma (Daoy) cancer cell lines. Interestingly, the dibrominated C18-acetylenic fatty acid (6) exhibited the most potent growth inhibitory activity against these cancer cell lines followed by Compounds 7 and 9. Apparently, the dibromination of the terminal olefinic moiety has an enhanced effect on the cytotoxic activity.
Studies on the Red Sea Sponge Haliclona sp. for its Chemical and Cytotoxic Properties.[Pubmed:27076747]
Pharmacogn Mag. 2016 Apr-Jun;12(46):114-9.
BACKGROUND: A great number of novel compounds with rich chemical diversity and significant bioactivity have been reported from Red Sea sponges. OBJECTIVE: To isolate, identify, and evaluate the cytotoxic activity of the chemical constituents of a sponge belonging to genus Haliclona collected from the Eastern coast of the Red Sea. MATERIALS AND METHODS: The total ethanolic extract of the titled sponge was subjected to intensive chromatographic fractionation and purification guided by cytotoxic bioassay toward various cancer cell lines. The structures of the isolated compounds were elucidated using spectroscopic techniques including one-dimension and two-dimension nuclear magnetic resonance, mass spectrometry, ultraviolet, and infrared data, as well as comparison with the reported spectral data for the known compounds. X-ray single-crystal structure determination was performed to determine the absolute configuration of compound 4. The screening of antiproliferative activity of the compounds was carried on three tumor cell lines, namely the human cervical cancer (HeLa), human hepatocellular carcinoma (HepG2), and human medulloblastoma (Daoy) cells using MTT assay. RESULTS: This investigation resulted in the isolation of a new indole alkaloid, 1-(1H-indol-3-yloxy) propan-2-ol (1), with the previously synthesized pyrrolidine alkaloid, (2R, 3S, 4R, 5R) pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4), isolated here from a natural source for the first time. In addition, six known compounds Tetillapyrone (2), norTetillapyrone (3), 2-methyl maleimide-5-oxime (5), maleimide-5-oxime (6), 5-(hydroxymethyl) dihydrofuran-2 (3H)-one (7), and ergosta-5,24 (28)-dien-3-ol (8) were also identified. Most of the isolated compounds exhibited weak cytotoxic activity against HepG-2, Daoy, and HeLa cancer cell lines. CONCLUSION: This is the first report of the occurrence of the indole and pyrrolidine alkaloids, 1-(1H-indol-2-yloxy) propan-2-ol (1), and the - (1-hydroxyethyl)-3,4-diol hydrochloride (4), in the Red Sea Haliclona sp. SUMMARY: From the Red Sea Haliclona sp. two alkaloids with indole and pyrrolidine nuclei, 1-(1H-indol-2-yloxy) propan-2-ol-(1) and pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4) were isolated and fully characterized; in addition to six known compounds (2, 3, 5-8)The absolute configuration and the three-dimension stereo-molecular structure of compound 4 were determined by X-ray crystallographyThe different extracts and isolated compounds showed weak cytotoxic activity against HepG-2, Daoy, and HeLa cancer cell lines.
New ceramides from the sponge Cinachyra cavernosa.[Pubmed:18696328]
J Asian Nat Prod Res. 2008 Jul-Aug;10(7-8):757-61.
Two new ceramides 1 and 2, and Tetillapyrone 3 have been isolated from the Indian sponge Cinachyra cavernosa. The structures of 1, 2, and 3 were determined by spectroscopic and chemical analyses.
Antifungal activity evaluation of the constituents of Haliclona baeri and Haliclona cymaeformis, collected from the Gulf of Thailand.[Pubmed:18463723]
Mar Drugs. 2007 Jun 25;5(2):40-51.
A new compound maleimide-5-oxime was isolated, together with 3,4-dihydroxybenzoic acid, Tetillapyrone, from the ethyl acetate extract of the marine sponge Haliclona baeri while Tetillapyrone, norTetillapyrone, p-hydroxybenzaldehyde and phenylacetic acid were isolated from the ethyl acetate extract of Haliclona cymaeformis, collected from the Gulf of Thailand. The structures of Tetillapyrone and norTetillapyrone were re-examined using HMBC correlations. Maleimide-5-oxime, Tetillapyrone and norTetillapyrone were found to be inactive against three human tumor cell lines (the estrogen-dependent ER(+) MCF-7, the estrogen-independent ER(-) MDA-MB-231 and NCI-H460. Maleimide-5-oxime, p-hydroxybenzaldehyde, phenylacetic acid, Tetillapyrone and norTetillapyrone were evaluated for their growth inhibitory effect against seven yeasts and eight filamentous fungi. Only norTetillapyrone showed antifungal activity, with a preponderance on the dermatophytic filamentous fungi.
Tetillapyrone and nortetillapyrone, two unusual hydroxypyran-2-ones from the marine sponge Tetilla japonica.[Pubmed:11520226]
J Nat Prod. 2001 Aug;64(8):1056-8.
Extraction of the marine sponge Tetilla japonica from the Bay of Thailand furnished Tetillapyrone and norTetillapyrone, two unusual tetrahydrofurylhydroxypyran-2-ones, whose structures were established by NMR spectrometry and an X-ray analysis of Tetillapyrone.


