ThalifendineCAS# 18207-71-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18207-71-1 | SDF | Download SDF |
| PubChem ID | 3084288 | Appearance | Powder |
| Formula | C19H16NO4 | M.Wt | 322.33 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
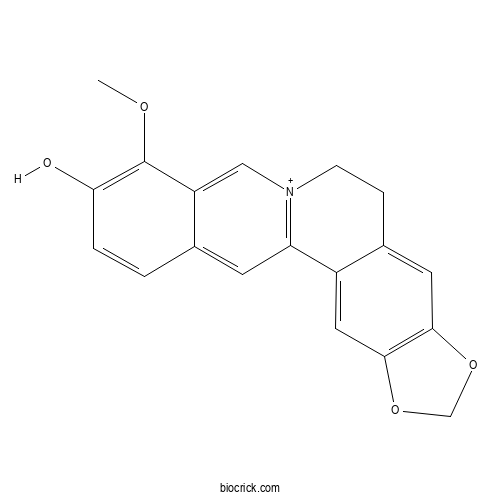
| Chemical Name | 16-methoxy-5,7-dioxa-13-azoniapentacyclo[11.8.0.02,10.04,8.015,20]henicosa-1(13),2,4(8),9,14,16,18,20-octaen-17-ol | ||
| SMILES | COC1=C(C=CC2=CC3=[N+](CCC4=CC5=C(C=C43)OCO5)C=C21)O | ||
| Standard InChIKey | OEGWOBMNQDATKP-UHFFFAOYSA-O | ||
| Standard InChI | InChI=1S/C19H15NO4/c1-22-19-14-9-20-5-4-12-7-17-18(24-10-23-17)8-13(12)15(20)6-11(14)2-3-16(19)21/h2-3,6-9H,4-5,10H2,1H3/p+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Thalifendine Dilution Calculator

Thalifendine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1024 mL | 15.5121 mL | 31.0241 mL | 62.0482 mL | 77.5603 mL |
| 5 mM | 0.6205 mL | 3.1024 mL | 6.2048 mL | 12.4096 mL | 15.5121 mL |
| 10 mM | 0.3102 mL | 1.5512 mL | 3.1024 mL | 6.2048 mL | 7.756 mL |
| 50 mM | 0.062 mL | 0.3102 mL | 0.6205 mL | 1.241 mL | 1.5512 mL |
| 100 mM | 0.031 mL | 0.1551 mL | 0.3102 mL | 0.6205 mL | 0.7756 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (2R,3S)-3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN0237
CAS No.:1966944-04-6
- 16,25-Diacetate cyclosiversioside F
Catalog No.:BCN0236
CAS No.:452919-90-3
- Fissistigine A
Catalog No.:BCN0235
CAS No.:70420-58-5
- Echinocystic acid 28-O-beta-D-glucoside
Catalog No.:BCN0234
CAS No.:99633-30-4
- 1-Methylhydantoin
Catalog No.:BCN0233
CAS No.:616-04-6
- 2-Naphthalenecarboxylic acid, 4-(D-glucopyranosyloxy)-1-hydroxy-3-(3-hydroxy-3-methylbutyl)-, methyl ester
Catalog No.:BCN0232
CAS No.:125906-48-1
- L-Gamma-Glutamyl-S-[(4-hydroxyphenyl)methyl]-L-cysteinylglycine
Catalog No.:BCN0231
CAS No.:129636-38-0
- Polygalasaponin II
Catalog No.:BCN0230
CAS No.:162857-62-7
- Valtrate Hydrine B4
Catalog No.:BCN0229
CAS No.:18296-48-5
- 24-Methylene cycloartanyl ferulate
Catalog No.:BCN0228
CAS No.:469-36-3
- Emodin 6-O-beta-D-glucoside
Catalog No.:BCN0227
CAS No.:34298-85-6
- Luteolin-7-O-alpha-L-arabinopyranosyl (1->6)-beta-D-glucopyranoside
Catalog No.:BCN0226
CAS No.:52714-82-6
- Licraside
Catalog No.:BCN0239
CAS No.:29913-71-1
- 5'-UMP disodium salt
Catalog No.:BCN0240
CAS No.:3387-36-8
- Norswertianolin
Catalog No.:BCN0241
CAS No.:54954-12-0
- Natsudaidain
Catalog No.:BCN0242
CAS No.:35154-55-3
- 3'-Hydroxyxanthyletin
Catalog No.:BCN0243
CAS No.:165900-08-3
- Demethylisoencecalin
Catalog No.:BCN0244
CAS No.:24672-84-2
- O-Desmethyl galanthamine
Catalog No.:BCN0245
CAS No.:60755-80-8
- Anhydrobyankangelicin
Catalog No.:BCN0246
CAS No.:35214-81-4
- Galanthamine 10-Oxide
Catalog No.:BCN0247
CAS No.:134332-50-6
- O-Acetylgalanthamine
Catalog No.:BCN0248
CAS No.:25650-83-3
- Dioscoreside C
Catalog No.:BCN0249
CAS No.:344912-80-7
- Dioscoreside E
Catalog No.:BCN0250
CAS No.:435321-73-6
Pharmacokinetics of Five Alkaloids and their Metabolites in Normal and Diabetic Rats after Oral Administration of Rhizoma coptidis.[Pubmed:34111890]
Planta Med. 2021 Jun 10.
Rhizoma coptidis has been clinically used for a long time for the treatment of various diseases in China, such as hypertension, diabetes, and inflammation. Previous studies have shown that alkaloid components of Rhizoma coptidis extract could be extensively metabolized and the metabolites were also considered to be the therapeutic material basis. However, until now, pharmacokinetic studies of the in vivo metabolites have not been revealed yet. The aim of the present study was to characterize the pharmacokinetics and excretions of five main alkaloids (berberine, jatrorrhizine, palmatine, epiberberine, and coptisine) and their seven metabolites (berberrubine, demethyleneberberine, jatrorrhizine-3-O-beta-D-glucuronide, Thalifendine-10-O-beta-D-glucuronide, berberrubine-9-O-beta-D-glucuronide, demethyleneberberine-2-O-sulfate, and demethyleneberberine-2-O-beta-D-glucuronide) in rats after oral administration of Rhizoma coptidis extract. Meanwhile, comparative pharmacokinetics and excretions of these analytes in diabetic model rats were also investigated, since Rhizoma coptidis is widely used for the treatment of diabetes. Our results showed that the in vivo existing forms of alkaloid components were phase II metabolites, highlighting the glucuronidation metabolic pathway. In diabetic model rats, the utilization of Rhizoma coptidis alkaloids was significantly increased and the biotransformation of berberine into berberrubine was significantly inhibited.
Quasi-Irreversible Inhibition of CYP2D6 by Berberine.[Pubmed:32987920]
Pharmaceutics. 2020 Sep 24;12(10). pii: pharmaceutics12100916.
In our previous study, Hwang-Ryun-Hae-Dok-Tang, which contains berberine (BBR) as a main active ingredient, inhibited cytochrome P450 (CYP) 2D6 in a quasi-irreversible manner. However, no information is available on the detailed mechanism of BBR-induced CYP2D6 inhibition. Thus, the present study aimed to characterize the inhibition mode and kinetics of BBR and its analogues against CYP2D6 using pooled human liver microsomes (HLM). BBR exhibited selective quasi-irreversible inhibition of CYP2D6 with inactivation rate constant (kinact) of 0.025 min(-1), inhibition constant (KI) of 4.29 microM, and kinact/KI of 5.83 mL/min/micromol. In pooled HLM, BBR was metabolized to Thalifendine (TFD), demethyleneberberine (DMB), M1 (proposed as demethylene-TFD), and to a lesser extent berberrubine (BRB), showing moderate metabolic stability with a half-life of 35.4 min and a microsomal intrinsic clearance of 7.82 microL/min/mg protein. However, unlike BBR, those metabolites (i.e., TFD, DMB, and BRB) were neither selective nor potent inhibitors of CYP2D6, based on comparison of half-maximal inhibitory concentration (IC50). Notably, TFD, but not DMB, exhibited metabolism-dependent CYP2D6 inhibition as in the case of BBR, which suggests that methylenedioxybenzene moiety of BBR may play a critical role in the quasi-irreversible inhibition. Moreover, the metabolic clearance of nebivolol (beta-blocker; CYP2D6 substrate) was reduced in the presence of BBR. The present results warrant further evaluation of BBR-drug interactions in clinical situations.
Rapid Identification of Berberine Metabolites in Rat Plasma by UHPLC-Q-TOF-MS.[Pubmed:31137649]
Molecules. 2019 May 24;24(10). pii: molecules24101994.
In this study, a reliable and rapid method based on ultra high performance liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry (UHPLC-Q-TOF-MS) technology and MetabolitePilot(MT) software was developed for berberine metabolites identification in rat plasma. The chemical structures of the metabolites and their product ions were tentatively characterized or identified according to the molecular weights detected and MS/MS data. In all, nine metabolites, including M1 (demethyleneberberine, C19H18NO4, m/z 324), M2 (glucuronic acid-conjugated demethyleneberberine, C25H26NO10, m/z 500), M3 (diglucuronide-conjugated demethyleneberberine, C31H34NO16, m/z 676), M4 (glucuronic acid-conjugated jatrorrhizine or glucuronic acid-conjugated columbamine, C26H28NO10, m/z 514), M5 (berberrubine or Thalifendine, C19H16NO4, m/z 322), M6 (glucuronic acid-conjugated berberrubine or glucuronic acid-conjugated Thalifendine, C25H24NO10, m/z 498), M7 (sulfite-conjugated berberrubine or sulfite-conjugated Thalifendine, C19H16NO7S, m/z 402), M8 (dihydroxy berberrubine or dihydroxy Thalifendine, C19H16NO6, m/z 354) and M9 (dihydroxy berberine, C20H18NO6, m/z 368) were tentatively characterized or identified. Several new deposition patterns and three new metabolites (M7, M8 and M9) are reported in this paper for the first time. This work not only provides significant insights into the understanding of the metabolic pathways of berberine, but also contributes in identifying potential active drug candidates from the metabolites.
Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity.[Pubmed:30442987]
Acta Pharmacol Sin. 2019 Jan;40(1):133-142.
Berberine, berberrubine, Thalifendine, demethyleneberberine, jatrorrhizine, and columbamine are six natural protoberberine alkaloid (PA) compounds that display extensive pharmacological properties and share the same protoberberine molecular skeleton with only slight substitution differences. The oral delivery of most PAs is hindered by their poor bioavailability, which is largely caused by P-glycoprotein (P-gp)-mediated drug efflux. Meanwhile, P-gp undergoes large-scale conformational changes (from an inward-facing to an outward-facing state) when transporting substrates, and these changes might strongly affect the P-gp-binding specificity. To confirm whether these six compounds are substrates of P-gp, to investigate the differences in efflux capacity caused by their trivial structural differences and to reveal the key to increasing their binding affinity to P-gp, we conducted a series of in vivo, in vitro, and in silico assays. Here, we first confirmed that all six compounds were substrates of P-gp by comparing the drug concentrations in wild-type and P-gp-knockout mice in vivo. The efflux capacity (net efflux) ranked as berberrubine > berberine > columbamine ~ jatrorrhizine > Thalifendine > demethyleneberberine based on in vitro transport studies in Caco-2 monolayers. Using molecular dynamics simulation and molecular docking techniques, we determined the transport pathways of the six compounds and their binding affinities to P-gp. The results suggested that at the early binding stage, different hydrophobic and electrostatic interactions collectively differentiate the binding affinities of the compounds to P-gp, whereas electrostatic interactions are the main determinant at the late release stage. In addition to hydrophobic interactions, hydrogen bonds play an important role in discriminating the binding affinities.
A new bisbenzylisoquinoline alkaloid isolated from Thalictrum foliolosum, as a potent inhibitor of DNA topoisomerase IB of Leishmania donovani.[Pubmed:26625837]
Fitoterapia. 2016 Mar;109:25-30.
Chemical investigation of the stem of Thalictrum foliolosum resulted in the isolation of two new bisbenzylisoquinoline alkaloids (1 and 2) along with known protoberberine group of isoquinoline alkaloids Thalifendine (3) and berberine (4). The structures of the new compounds were established by detailed 2D NMR spectral analysis with their configurations determined from their optical rotation values and confirmed using circular dichroism. Inhibitory activities of these four compounds against DNA topoisomerase IB of Leishmania donovani were evaluated. Compound 2 exhibited almost complete inhibition of the enzyme activity at 50 muM concentration and it was found to be effective in killing both wild type as well as SAG resistant promastigotes of the parasite.
Rapid screening and distribution of bioactive compounds in different parts of Berberis petiolaris using direct analysis in real time mass spectrometry.[Pubmed:29403947]
J Pharm Anal. 2015 Oct;5(5):332-335.
Berberis petiolaris Wall. ex G. Don, an unexplored medicinal plant belonging to the family Berberidaceae, is a large deciduous shrub found in Western Himalaya between 1800-3000 m. Chemical profiling of fruit, leaf, root and stem was done by direct analysis in real time mass spectrometry followed by multivariate analysis for discrimination among the plant parts. The bioactive compounds, including magnoflorine, berberine, jatrorrhizine, Thalifendine/berberrubine, demethyleneberberine, reticuline, 8-oxoberberine, N-methyltetrahydroberberine, tetrahydropalmatine, tetrahydroberberine and palmatine, were identified by their exact mass measurement and the corresponding molecular formula of each compound. A comparative study of distribution pattern for all these bioactive alkaloids showed qualitative and quantitative variations in different parts of B. petiolaris. Principal component analysis clearly discriminated each part of B. petiolaris plant.
Differential inhibition of CYP1-catalyzed regioselective hydroxylation of estradiol by berberine and its oxidative metabolites.[Pubmed:26403084]
Drug Metab Pharmacokinet. 2015 Oct;30(5):374-83.
Berberine is a pharmacologically active alkaloid present in widely used medicinal plants, such as Coptis chinensis (Huang-Lian). The hormone estradiol is oxidized by cytochrome P450 (CYP) 1B1 to primarily form the genotoxic metabolite 4-hydroxyestradiol, whereas CYP1A1 and CYP1A2 predominantly generate 2-hydroxyestradiol. To illustrate the effect of berberine on the regioselective oxidation of estradiol, effects of berberine and its metabolites on CYP1 activities were studied. Among CYP1s, CYP1B1.1, 1.3 (L432V), and 1.4 (N453S)-catalyzed 4-hydroxylation were preferentially inhibited by berberine. Differing from the competitive inhibition of CYP1B1.1 and 1.3, N453S substitution in CYP1B1 allowed a non-competitive or mixed-type pattern. An N228T in CYP1B1 highly decreased its activity and preference to 4-hydroxylation. A reverse mutation of T223N in CYP1A2 retained its 2-hydroxylation preference, but enhanced its inhibition susceptibility to berberine. Compared with berberine, metabolites demethyleneberberine and Thalifendine caused weaker inhibition of CYP1A1 and CYP1B1 activities. Unexpectedly, Thalifendine was more potent than berberine in the inhibition of CYP1A2, in which case an enhanced interaction through polar hydrogen-pi bond was predicted from the docking analysis. These results demonstrate that berberine preferentially inhibits the estradiol 4-hydroxylation activity of CYP1B1 variants, suggesting that 4-hydroxyestradiol-mediated toxicity might be reduced by berberine, especially in tissues/tumors highly expressing CYP1B1.
The Effect of Oxidation on Berberine-Mediated CYP1 Inhibition: Oxidation Behavior and Metabolite-Mediated Inhibition.[Pubmed:25953522]
Drug Metab Dispos. 2015 Jul;43(7):1100-7.
The protoberberine alkaloid berberine carries methylenedioxy moiety and exerts a variety of pharmacological effects, such as anti-inflammation and lipid-lowering effects. Berberine causes potent CYP1B1 inhibition, whereas CYP1A2 shows resistance to the inhibition. To reveal the influence of oxidative metabolism on CYP1 inhibition by berberine, berberine oxidation and the metabolite-mediated inhibition were determined. After NADPH-fortified preincubation of berberine with P450, the inhibition of CYP1A1 and CYP1B1 variants (CYP1B1.1, CYP1B1.3, and CYP1B1.4) by berberine was not enhanced, and CYP1A2 remained resistant. Demethyleneberberine was identified as the most abundant metabolite of CYP1A1- and CYP1B1-catalyzed oxidations, and Thalifendine was generated at a relatively low rate. CYP1A1-catalyzed berberine oxidation had the highest maximal velocity (V max) and exhibited positive cooperativity, suggesting the assistance of substrate binding when the first substrate was present. In contrast, the demethylenation by CYP1B1 showed the property of substrate inhibition. CYP1B1-catalyzed berberine oxidation had low K m values, but it had V max values less than 8% of those of CYP1A1. The dissociation constants generated from the binding spectrum and fluorescence quenching suggested that the low K m values of CYP1B1-catalyzed oxidation might include more than the rate constants describing berberine binding. The natural protoberberine/berberine fmetabolites with methylenedioxy ring-opening (palmatine, jatrorrhizine, and demethyleneberberine) and the demethylation (Thalifendine and berberrubine) caused weak CYP1 inhibition. These results demonstrated that berberine was not efficiently oxidized by CYP1B1, and metabolism-dependent irreversible inactivation was minimal. Metabolites of berberine caused a relatively weak inhibition of CYP1.
Histochemical evaluation of alkaloids in rhizome of Coptis chinensis using laser microdissection and liquid chromatography/mass spectrometry.[Pubmed:25209714]
Drug Test Anal. 2015 Jun;7(6):519-30.
Traditional macroscopic and microscopic identification methods of medicinal materials are economical and practical, but usually experience-based due to few chemical supports. Here histochemical evaluation on bioactive components of Coptidis Rhizoma (CR) in anatomic sections using laser microdissection and liquid chromatography-mass spectrometry (LMD-LC-MS) was developed to correlate the inner quality and outer features of materials from different growing areas. Results of a total 33 peaks representing potential different alkaloids were detected and 8 common peaks were identified as the major alkaloids, namely magnoflorine, Thalifendine, columbamine, epiberberine, jatrorrhizine, coptisine, palmatine, and berberine. Six major alkaloids were quantified in the top and middle sections of raw materials and in their tissues and cells at the same time. Histochemical analyses showed consistent results with direct determination in raw materials and explained the reason why top sections of all samples contained higher contents of alkaloids by giving out attributions of each alkaloid in different anatomic sections. Besides, results manifested the distribution and accumulation rules of alkaloids in diverse tissues and cells of CR. This study demonstrates an effective and scientific way to correlate bioactive components and morphological features of medicinal materials, which is beneficial to future research, agriculture and application.
Tissue distribution of berberine and its metabolites after oral administration in rats.[Pubmed:24205048]
PLoS One. 2013 Oct 31;8(10):e77969.
Berberine (BBR) has been confirmed to have multiple bioactivities in clinic, such as cholesterol-lowering, anti-diabetes, cardiovascular protection and anti- inflammation. However, BBR's plasma level is very low; it cannot explain its pharmacological effects in patients. We consider that the in vivo distribution of BBR as well as of its bioactive metabolites might provide part of the explanation for this question. In this study, liquid chromatography coupled to ion trap time-of-flight mass spectrometry (LC/MS(n)-IT-TOF) as well as liquid chromatography that coupled with tandem mass spectrometry (LC-MS/MS) was used for the study of tissue distribution and pharmacokinetics of BBR in rats after oral administration (200 mg/kg). The results indicated that BBR was quickly distributed in the liver, kidneys, muscle, lungs, brain, heart, pancreas and fat in a descending order of its amount. The pharmacokinetic profile indicated that BBR's level in most of studied tissues was higher (or much higher) than that in plasma 4 h after administration. BBR remained relatively stable in the tissues like liver, heart, brain, muscle, pancreas etc. Organ distribution of BBR's metabolites was also investigated paralleled with that of BBR. Thalifendine (M1), berberrubine (M2) and jatrorrhizine (M4), which the metabolites with moderate bioactivity, were easily detected in organs like the liver and kidney. For instance, M1, M2 and M4 were the major metabolites in the liver, among which the percentage of M2 was up to 65.1%; the level of AUC (0-t) (area under the concentration-time curve) for BBR or the metabolites in the liver was 10-fold or 30-fold higher than that in plasma, respectively. In summary, the organ concentration of BBR (as well as its bioactive metabolites) was higher than its concentration in the blood after oral administration. It might explain BBR's pharmacological effects on human diseases in clinic.
In vitro metabolic study of Rhizoma coptidis extract using liver microsomes immobilized on magnetic nanoparticles.[Pubmed:24048516]
Anal Bioanal Chem. 2013 Nov;405(27):8807-17.
Although metabolic study of individual active compounds isolated from herbal plants has been intensive, it cannot truly reflect the fate of herbs because the herbal extracts in use have many constituents. To address this problem, whole extracts of herbs should be investigated. Microsomes have been heavily used in the in vitro metabolic study of drugs, and various materials have been used to immobilize microsomes to develop highly effective and reusable bioreactors in this field. In this work, rat liver microsomes were immobilized on magnetic nanoparticles (LMMNPs) to develop a highly active and recoverable nanoparticle bioreactor. Using this bioreactor, we investigated the in vitro metabolism of Rhizoma coptidis extract. Incubation of berberine, a major active ingredient of R. coptidis, with LMMNPs for 20 min produced two metabolites, i.e., demethyleneberberine and Thalifendine, at high levels. From a comparison of the time courses of Thalifendine formation obtained by ultraperformance liquid chromatography-mass spectrometry analysis, it was found that LMMNPs had a higher biological activity than free liver microsomes in metabolizing berberine. Further, the activity of LMMNPs remained almost unchanged after six consecutive uses in the incubation tests. Metabolism of R. coptidis extracts by LMMNPs was studied. The same two metabolites of berberine, i.e., demethyleneberberine and Thalifendine, were detected. After a thorough study seeking support for this observation, it was found that demethyleneberberine was the common metabolite of five protoberberine-type alkaloids present in R. coptidis extract, including palmatine, jatrorrhizine, columbanine, epiberberine, and berberine.
Excretion of berberine and its metabolites in oral administration in rats.[Pubmed:24006193]
J Pharm Sci. 2013 Nov;102(11):4181-92.
Berberine (BBR) has been confirmed to show extensive bioactivities for the treatments of diabetes and hypercholesterolemia in clinic. However, there are few pharmacokinetic studies to elucidate the excretions of BBR and its metabolites. Our research studied the excretions of BBR and its metabolites in rats after oral administration (200 mg/kg). Metabolites in bile, urine, and feces were detected by liquid chromatography coupled to ion trap time-of-flight mass spectrometry; meanwhile, a validated liquid chromatography coupled with tandem mass spectrometry method was developed for their quantifications. Sixteen metabolites, including 10 Phase I and six Phase II metabolites were identified and clarified after dosing in vivo. Total recovered rate of BBR was 22.83% (19.07% of prototype and 3.76% of its metabolites) with 9.2 x 10(-6) % in bile (24 h), 0.0939% in urine (48 h), and 22.74% in feces (48 h), respectively. 83% of BBR was excreted as Thalifendine (M1) from bile, whereas Thalifendine (M1) and berberrubine (M2) were the major metabolites occupying 78% of urine excretion. Most of BBR and its metabolites were found in feces containing 84% of prototype. In summary, we provided excretion profiles of BBR and its metabolites after oral administration in rats in vivo.
Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes.[Pubmed:21569619]
J Transl Med. 2011 May 15;9:62.
BACKGROUND: Berberine (BBR) is a drug with multiple effects on cellular energy metabolism. The present study explored answers to the question of which CYP450 (Cytochrome P450) isoenzymes execute the phase-I transformation for BBR, and what are the bioactivities of its metabolites on energy pathways. METHODS: BBR metabolites were detected using LC-MS/MS. Computer-assistant docking technology as well as bioassays with recombinant CYP450s were employed to identify CYP450 isoenzymes responsible for BBR phase-I transformation. Bioactivities of BBR metabolites in liver cells were examined with real time RT-PCR and kinase phosphorylation assay. RESULTS: In rat experiments, 4 major metabolites of BBR, berberrubine (M1), Thalifendine (M2), demethyleneberberine (M3) and jatrorrhizine (M4) were identified in rat's livers using LC-MS/MS (liquid chromatography-tandem mass spectrometry). In the cell-free transformation reactions, M2 and M3 were detectable after incubating BBR with rCYP450s or human liver microsomes; however, M1 and M4 were below detective level. CYP2D6 and CYP1A2 played a major role in transforming BBR into M2; CYP2D6, CYP1A2 and CYP3A4 were for M3 production. The hepatocyte culture showed that BBR was active in enhancing the expression of insulin receptor (InsR) and low-density-lipoprotein receptor (LDLR) mRNA, as well as in activating AMP-activated protein kinase (AMPK). BBR's metabolites, M1-M4, remained to be active in up-regulating InsR expression with a potency reduced by 50-70%; LDLR mRNA was increased only by M1 or M2 (but not M3 and M4) with an activity level 35% or 26% of that of BBR, respectively. Similarly, AMPK-alpha phosphorylation was enhanced by M1 and M2 only, with a degree less than that of BBR. CONCLUSIONS: Four major BBR metabolites (M1-M4) were identified after phase-I transformation in rat liver. Cell-free reactions showed that CYP2D6, CYP1A2 and CYP3A4 seemed to be the dominant CYP450 isoenzymes transforming BBR into its metabolites M2 and M3. BBR's metabolites remained to be active on BBR's targets (InsR, LDLR, and AMPK) but with reduced potency.


