NatsudaidainCAS# 35154-55-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35154-55-3 | SDF | Download SDF |
| PubChem ID | 3084605 | Appearance | Powder |
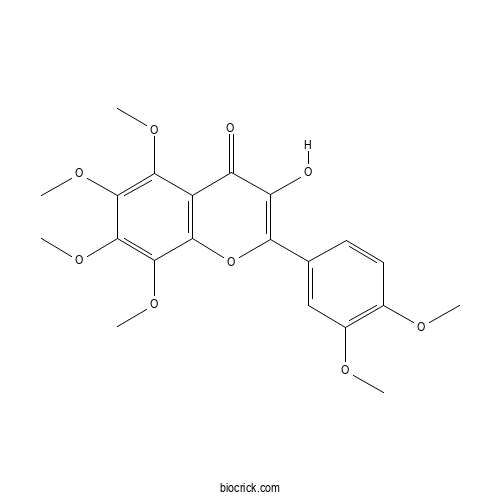
| Formula | C21H22O9 | M.Wt | 418.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dimethoxyphenyl)-3-hydroxy-5,6,7,8-tetramethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(O2)C(=C(C(=C3OC)OC)OC)OC)O)OC | ||
| Standard InChIKey | CCJBNIRSVUKABH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H22O9/c1-24-11-8-7-10(9-12(11)25-2)16-15(23)14(22)13-17(26-3)19(27-4)21(29-6)20(28-5)18(13)30-16/h7-9,23H,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Natsudaidain Dilution Calculator

Natsudaidain Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3901 mL | 11.9503 mL | 23.9006 mL | 47.8011 mL | 59.7514 mL |
| 5 mM | 0.478 mL | 2.3901 mL | 4.7801 mL | 9.5602 mL | 11.9503 mL |
| 10 mM | 0.239 mL | 1.195 mL | 2.3901 mL | 4.7801 mL | 5.9751 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.956 mL | 1.195 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Norswertianolin
Catalog No.:BCN0241
CAS No.:54954-12-0
- 5'-UMP disodium salt
Catalog No.:BCN0240
CAS No.:3387-36-8
- Licraside
Catalog No.:BCN0239
CAS No.:29913-71-1
- Thalifendine
Catalog No.:BCN0238
CAS No.:18207-71-1
- (2R,3S)-3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN0237
CAS No.:1966944-04-6
- 16,25-Diacetate cyclosiversioside F
Catalog No.:BCN0236
CAS No.:452919-90-3
- Fissistigine A
Catalog No.:BCN0235
CAS No.:70420-58-5
- Echinocystic acid 28-O-beta-D-glucoside
Catalog No.:BCN0234
CAS No.:99633-30-4
- 1-Methylhydantoin
Catalog No.:BCN0233
CAS No.:616-04-6
- 2-Naphthalenecarboxylic acid, 4-(D-glucopyranosyloxy)-1-hydroxy-3-(3-hydroxy-3-methylbutyl)-, methyl ester
Catalog No.:BCN0232
CAS No.:125906-48-1
- L-Gamma-Glutamyl-S-[(4-hydroxyphenyl)methyl]-L-cysteinylglycine
Catalog No.:BCN0231
CAS No.:129636-38-0
- Polygalasaponin II
Catalog No.:BCN0230
CAS No.:162857-62-7
- 3'-Hydroxyxanthyletin
Catalog No.:BCN0243
CAS No.:165900-08-3
- Demethylisoencecalin
Catalog No.:BCN0244
CAS No.:24672-84-2
- O-Desmethyl galanthamine
Catalog No.:BCN0245
CAS No.:60755-80-8
- Anhydrobyankangelicin
Catalog No.:BCN0246
CAS No.:35214-81-4
- Galanthamine 10-Oxide
Catalog No.:BCN0247
CAS No.:134332-50-6
- O-Acetylgalanthamine
Catalog No.:BCN0248
CAS No.:25650-83-3
- Dioscoreside C
Catalog No.:BCN0249
CAS No.:344912-80-7
- Dioscoreside E
Catalog No.:BCN0250
CAS No.:435321-73-6
- 4'-O-Methyllucenin II (Diosmetin 6,8-di-C-glucoside)
Catalog No.:BCN0251
CAS No.:98813-28-6
- Gynosaponin TN2
Catalog No.:BCN0252
CAS No.:77658-95-8
- Peiioside B
Catalog No.:BCN0253
CAS No.:1610618-91-1
- Euphorbia factor L25
Catalog No.:BCN0254
CAS No.:303174-98-3
Online extraction-high performance liquid chromatography-diode array detector-quadrupole time-of-flight tandem mass spectrometry for rapid flavonoid profiling of Fructus aurantii immaturus.[Pubmed:29413571]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 1;1077-1078:1-6.
Chemical profiling of natural products by high performance liquid chromatography (HPLC) was critical for understanding of their clinical bioactivities, and sample pretreatment steps have been considered as a bottleneck for analysis. Currently, concerted efforts have been made to develop sample pretreatment methods with high efficiency, low solvent and time consumptions. Here, a simple and efficient online extraction (OLE) strategy coupled with HPLC-diode array detector-quadrupole time-of-flight tandem mass spectrometry (HPLC-DAD-QTOF-MS/MS) was developed for rapid chemical profiling. For OLE strategy, guard column inserted with ground sample (2mg) instead of sample loop was connected with manual injection valve, in which components were directly extracted and transferred to HPLC-DAD-QTOF-MS/MS system only by mobile phase without any extra time, solvent, instrument and operation. By comparison with offline heat-reflux extraction for Fructus aurantii immaturus (Zhishi), OLE strategy presented higher extraction efficiency perhaps because of the high pressure and gradient elution mode. A total of eighteen flavonoids were detected according to their retention times, UV spectra, exact mass, and fragmentation ions in MS/MS spectra, and compound 9, Natsudaidain-3-O-glucoside, was discovered in Zhishi for the first time. It is concluded that the developed OLE-HPLC-DAD-QTOF-MS/MS system offers new perspectives for rapid chemical profiling of natural products.
Permeation of Polymethoxyflavones into the Mouse Brain and Their Effect on MK-801-Induced Locomotive Hyperactivity.[Pubmed:28245567]
Int J Mol Sci. 2017 Feb 24;18(3). pii: ijms18030489.
Accumulating data have indicated that citrus polymethoxyflavones (PMFs) have the ability to affect brain function. In the present study, we showed that 3,5,6,7,8,3',4'-heptamethoxy- flavone (HMF) given intraperitoneally to mice was immediately detected in the brain and that the permeability of the brain tissues to it was significantly higher than that of other citrus PMFs (nobiletin, tangeretin, and Natsudaidain). The permeation of these PMFs into the brain well correlated with their abilities to suppress MK-801-induced locomotive hyperactivity, suggesting that HMF had the ability to act directly in the brain. We also obtained data suggesting that the suppressive effect of HMF on MK-801-induced locomotive hyperactivity was mediated by phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) in the hippocampus.
[Isolation and structural identification of flavonoids from Aurantii Fructus].[Pubmed:26591524]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(12):2352-6.
Aurantii Fructus is the dried and immature fruit of Citrus aurantium and its cultivars. To investigate the chemical constituents of Aurantii Fructus, the separation and purification of constituents were performed by column chromatography on silica gel LH-20, HW-40, ODS, PHPLC and PTLC. Fourteen flavonoids, including four flavone glycosides and ten polymethoxyflavones (PMFs) were isolated from the EtOAc fraction and Petroleum ether fraction of Aurantii Fructus and their structures were identified by physicochemical properties and spectral data (NMR and MS) as (2R) -and (2S)-6"-O-acetylprunin (1,2), naringenin-7-O-beta-D-glucopyranside (3), 5,7,4'-trihydroxy-8,3'-dimethoxyflavone-3-O-6"-(3-hydroxyl-3-methylglutaroyl)-bet a-D-glucopyranoside(4), 4'-hydroxy-5,6, 7-trimethoxyflavone (5), Natsudaidain (6), nobiletin (7), sinensetin (8), 5,6,7,4'-tetramethoxyflavone (9), 5,7,8,4'-tetramethoxyflavone (10), 3,5,6,7,8,3',4'-heptamethoxyflavone (11), tangeretin (12), 5-demethyl nobiletin (13), and 5-hydroxy-6,7,3', 4'-tetramethoxyflavone (14). Compound 3-5 s were isolated from this plant for the first time and compound 1 was a new one.
Effect of natsudaidain isolated from Citrus plants on TNF-alpha and cyclooxygenase-2 expression in RBL-2H3 cells.[Pubmed:19126304]
J Pharm Pharmacol. 2009 Jan;61(1):109-14.
OBJECTIVES: Flavonoids inhibit the activity of chemical mediators released from mast cells. Our aim was to investigate the effects of Natsudaidain, a polymethoxyflavone isolated from Citrus plants, on mast cells. METHODS: We investigated the inhibitory effects of Natsudaidain, which is a polymethoxyflavone isolated from Citrus plants, on histamine release, tumour necrosis factor-alpha production and cyclooxygenase-2 expression in Ca ionophore-stimulated rat basophilic leukemia cells (A23187-stimulated RBL-2H3 cells) by spectrofluorometric, ELISA and immunoblotting methods. KEY FINDINGS: The percent of histamine release from A23187-stimulated RBL-2H3 cells pretreated with Natsudaidain at 5, 25 and 50 microM was not changed as compared with non-treated A23187-stimulated cells. At 100 and 200 microM, Natsudaidain pretreatment resulted in slightly reduced histamine release (% histamine release, 89.8+/-3.5% and 71.5+/-5.6% at 100 and 200 microM). Thus, Natsudaidain hardly affects histamine release from RBL-2H3 cells, except at high concentrations. On the other hand, Natsudaidain dose-dependently inhibited tumour necrosis factor-alpha protein and mRNA levels in A23187-stimulated RBL-2H3 cells; a concentration of 6.8 microM was required for a 50% reduction. In addition, all concentrations of this compound that we tested also inhibited cyclooxygenase-2 protein expression. The mRNA levels of cyclooxygenase-2 in A23187-stimulated RBL-2H3 cells treated with Natsudaidain were also markedly decreased. The phosphorylated-p38 MAPK protein levels in A23187-stimulated RBL-2H3 cells treated with Natsudaidain were lower than in the non-treated cells. CONCLUSIONS: These findings suggest that Natsudaidain inhibits tumour necrosis factor-alpha and cyclooxygenase-2 production by suppressing p38 MAPK phosphorylation but not p65 NFkappaB phosphorylation, and that Natsudaidain might alleviate inflammatory diseases.
A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells.[Pubmed:18053806]
Biochem Biophys Res Commun. 2008 Feb 1;366(1):168-73.
The activation of mitogen-activated protein/extracellular signal-regulated kinase (MEK) is well known to be associated with tumor invasion and metastasis. We previously reported that a polymethoxyflavonoid, nobiletin (5,6,7,8,3',4'-hexamethoxyflavone), derived from Citrus depressa (Hayata), inhibits the phosphorylation of MEK and thereby suppresses matrix metalloproteinase (MMP) expression in a tumor-metastasis stimulator, 12-O-tetradecanoyl phorbol 13-acetate (TPA)-stimulated human fibrosarcoma HT-1080 cells [Mol. Cancer Ther. 3 (2004) 839-847]. In the present study, we investigated whether or not nobiletin might directly influence MEK activity to exhibit the antitumor metastatic activity in vitro. MEK kinase assay using myelin basic protein (MBP) revealed that TPA-augmented MEK activity in HT-1080 cells and that the augmented MEK activity was diminished by nobiletin treatment. In addition, the decrease in MEK activity caused by nobiletin was found to inhibit the phosphorylation of extracellular regulated kinases (ERK), a downstream signaling factor for MEK. Furthermore, when an immunoprecipitated active MEK was incubated with nobiletin under cell-free conditions, nobiletin was found to inhibit the MEK-mediated MBP phosphorylation. In contrast, other citrus polymethoxyflavonoids such as 3-hydroxy-5,6,7,8,3',4'-hexamethoxyflavone (Natsudaidain) and 3,5,6,7,8,3',4'-heptamethoxyflavone, did not directly inhibit MEK activity. Moreover, Natsudaidain and 3,5,6,7,8,3',4'-heptamethoxyflavone exhibited no or less inhibitory effect than nobiletin on the proMMP-9/progelatinase B production in HT-1080 cells. Therefore, these results provide novel evidence that nobiletin directly inhibits MEK activity and decreases the sequential phosphorylation of ERK, exhibiting the antitumor metastatic activity by suppressing MMP expression in HT-1080 cells.
Quantitative study of fruit flavonoids in citrus hybrids of King (C. nobilis) and Mukaku Kishu (C. kinokuni).[Pubmed:11513699]
J Agric Food Chem. 2001 Aug;49(8):3982-6.
Twenty-four Citrus hybrids of King (C. nobilis) and Mukaku Kishu (C. kinokuni) were examined for their flavonoid profiles of the edible part by reversed-phase HPLC analysis. Two hybrids (G-155 and G-156) contained higher amounts of Natsudaidain than their parents, whereas the remainder of the hybrids had a character intermediate between those of King and Mukaku Kishu on the basis of polymethoxylated flavone composition. Principal component analysis revealed the distribution of the hybrids by quantifying 23 flavonoid contents.
HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from citrus juices.[Pubmed:10563860]
J Agric Food Chem. 1999 Jan;47(1):128-35.
Citrus plants are rich sources of various bioactive flavonoids. To eliminate masking effects caused by hesperidin, naringin, and neoeriocitrin, the abundant flavonoid glycosides which make up 90% of the conventionally prepared sample, the readily extractable fraction from Citrus juice was prepared by adsorbing on HP-20 resin and eluting with EtOH and acetone from the resin and was subjected to HL-60 differentiation assay and quantitative analysis of major flavonoids. Screening of 34 Citrus juices indicated that King (C. nobilis) had a potent activity for inducing differentiation of HL-60, and the active principles were isolated and identified as four polymethoxylated flavonoids, namely, nobiletin, 3,3',4',5,6,7, 8-heptamethoxyflavone, Natsudaidain, and tangeretin. HPLC analysis of the readily extractable fraction also indicated that King contained high amounts of these polymethoxylated flavonoids among the Citrus juices examined. Principal component and cluster analyses of the readily extractable flavonoids indicated peculiarities of King and Bergamot.
Structure-activity relationship of cardiotonic flavonoids in guinea-pig papillary muscle.[Pubmed:10404425]
J Ethnopharmacol. 1999 Jun;65(3):267-72.
Sixteen flavonoids were tested for a positive inotropic effect (PIE) on guinea-pig papillary muscle paced at 0.2 Hz in a Krebs-Henseleit solution at 30 degrees C. The structure-activity relationship was investigated by determining both the pD2 value and the intrinsic activity in the case of ten flavonols, three flavones, one flavanone and two catechins. Quercetin showed the most potent intrinsic activity, and produced the strongest inotropic responses among the 16 compounds. The relative order of potency of the tested flavonoids was quercetin > morin = kaempferol = HEPTA > luteolin = apigenin > Natsudaidain = fisetin = galangin. Those that did not produce any PIE were 3-hydroxyflavone, flavone, glycosides of quercetin (rutin and hyperin), flavanones (naringenin) and catechins. With respect to the essential flavonoid nucleus for PIE development, the presence of a hydroxy group at C-4', an alpha, beta-unsaturated ketone on the C-ring and a reasonable lipophilic moiety in the molecule are required. Pharmacological analyses suggest that there is a common mechanism for the PIE and it is cyclic AMP dependent.
Antiproliferative activity of flavonoids on several cancer cell lines.[Pubmed:10380632]
Biosci Biotechnol Biochem. 1999 May;63(5):896-9.
Twenty-seven Citrus flavonoids were examined for their antiproliferative activities against several tumor and normal human cell lines. As a result, 7 flavonoids were judged to be active against the tumor cell lines, while they had weak antiproliferative activity against the normal human cell lines. The rank order of potency was luteolin, Natsudaidain, quercetin, tangeretin, eriodictyol, nobiletin, and 3,3',4',5,6,7,8-heptamethoxyflavone. The structure-activity relationship established from comparison among these flavones and flavanones showed that the ortho-catechol moiety in ring B and a C2-C3 double bond were important for the antiproliferative activity. As to polymethoxylated flavones, C-3 hydroxyl and C-8 methoxyl groups were essential for high activity.
Effect of citrus flavonoids on HL-60 cell differentiation.[Pubmed:10368686]
Anticancer Res. 1999 Mar-Apr;19(2A):1261-9.
Twenty-seven Citrus flavonoids were examined for their activity of induction of terminal differentiation of human promyelocytic leukemia cells (HL-60) by nitro blue tetrazolium (NBT) reducing, nonspecific esterase, specific esterase, and phagocytic activities. 10 flavonoids were judged to be active (percentage of NBT reducing cells was more than 40% at a concentration of 40 microM), and the rank order of potency was Natsudaidain, luteolin, tangeretin, quercetin, apigenin, 3, 3, '4, '5, 6, 7, 8-heptamethoxyflavone, nobiletin, acacetin, eriodictyol, and taxifolin. These flavonoids exerted their activity in a dose-dependent manner. HL-60 cells treated with these flavonoids differentiated into mature monocyte/macrophage. The structure-activity relationship established from comparison between flavones and flavanones revealed that ortho-catechol moiety in ring B and C2-C3 double bond had an important role for induction of differentiation of HL-60. In polymethoxylated flavones, hydroxyl group at C3 and methoxyl group at C8 enhanced the differentiation-inducing activity.
Cardiotonic flavonoids from Citrus plants (Rutaceae).[Pubmed:7703977]
Biol Pharm Bull. 1994 Nov;17(11):1519-21.
Two flavonoids, 3,5,6,7,8,3',4'-heptamethoxyflavone (HEPTA) and Natsudaidain isolated from Citrus plants (Rutaceae), produced a positive inotropic effect (PIE) on guinea-pig papillary muscle. Natsudaidain (pD2 4.98 +/- 0.07) was more potent than HEPTA (pD2 4.33 +/- 0.08), but the maximum PIE of HEPTA was greater than that of Natsudaidain. The PIE of HEPTA was completely inhibited by reserpinization of the guinea pig, and partially inhibited by metoprolol and carbachol. The carbachol inhibition was omitted by atropine. The mechanism of PIE of HEPTA is accounted for catecholamine release from cardiac tissue.


