Aconitum flavum
Aconitum flavum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Aconitum flavum
- Cat.No. Product Name CAS Number COA
-
BCN2800
12-Epinapelline110064-71-6
Instructions
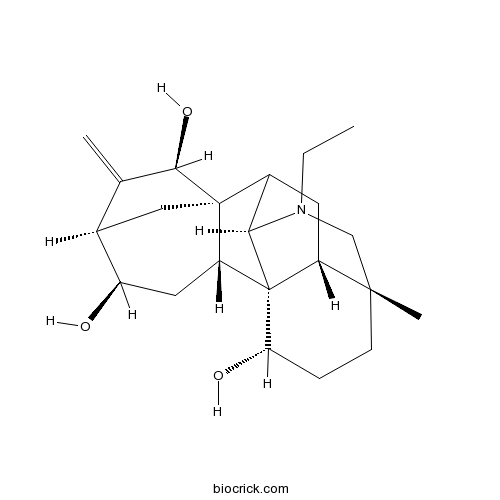
-
BCN1014
Aconitine302-27-2
Instructions
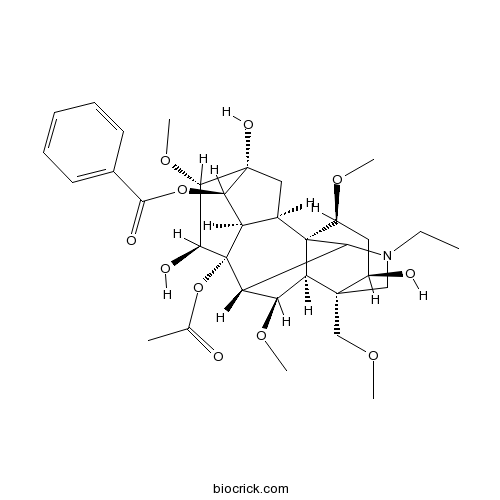
-
BCN2797
3-Deoxyaconitine3175-95-9
Instructions

[Study on baking processing technology of hui medicine Aconitum flavum].[Pubmed: 25090673]
To screen and optimize the processing technology of Aconitum flavum.
[Study on chemical components of Aconitum flavum and their inflammatory and analgesic effects].[Pubmed: 24218966]
To study the chemical compositions of active site of Aconitum flavum and their pharmacological effects on anti-inflammatory andantalgic.
Neoline from Aconitum flavum Hand.[Pubmed: 21754813]
The title compound, C(24)H(39)NO(6) [systematic name: (1α,6α,14α,16β)-N-ethyl-6,16-dimeth-oxy-4-meth-oxy-methylaconitane-1,8,14-triol], is a C(19)-diterpenoid alkaloid from the roots of Aconitum flavum Hand. The mol-ecule has an aconitane carbon skeleton with four six-membered rings and two five-membered rings. Both five-membered rings adopt envelope conformations. Two six-membered rings adopt chair conformations, whereas the other two adopt boat conformations. Intra-molecular O-H⋯O and O-H⋯N and inter-molecular O-H⋯O hydrogen bonds are present in the structure. In the crystal, one methyl group is disordered over two sites with an occupancy ratio of 0.70 (3):0.30 (3).
[Optimization of extraction process of total alkaloids from radix of Aconitum flavum using response surface methodology].[Pubmed: 21355203]
To study the technological parameters of the extraction process of total alkaloids from Radix of Aconitum flavum.
Development of natural products as drugs acting on central nervous system.[Pubmed: 1841995]
We have recently studied several natural product constituents which have effects on the CNS. (1) Tetrahydropalmatine (THP) and its analogues were isolated from Corydalis ambigua and various species of Stephania. (+)-THP and (-)-THP possess not only analgesic activity, but also exert sedative-tranquilizing and hypnotic actions. Results of receptor binding assay and their pre- and post-synaptic effects on dopaminergic system indicate that (-)-THP and (-)-stepholidine are dopamine receptor antagonists while (+)-THP is a selective dopamine depletor. (2) 3-Acetylaconitine (AAC) is an alkaloid isolated from Aconitum flavum. The relative potency of analgesic action of AAC was 5.1-35.6 and 1250-3912 times that of morphine and aspirin, respectively. The analgesic effect of AAC was not antagonized by naloxone, but was eliminated by reserpine. In monkeys, after AAC was injected for 92 days, no abstinence syndrome was seen after sudden AAC withdrawal or when challenged with nalorphine. (3) Huperzine A (Hup-A) is an alkaloid isolated from Huperzia serrata which was found to be a selective ChE inhibitor and could improve learning and retrieval processes. Preliminary clinical studies showed that Hup-A improve short- and long-term memory in patients of cerebral arteriosclerosis with memory impairment. (4) Ranamargarin is a new tetradecapeptide isolated from the skin of the Chinese frog Rana margaratae. This peptide may mainly act on NK-1 receptor.
Structures of Flavamine and Flavadine from Aconitum flavum.[Pubmed: 17265275]
Two new diterpenoid alkaloids, flavamine ( 1) and flavadine ( 2), were isolated from the roots of ACONITUM FLAVUM Hand-Mazz. The structures were established on the bases of spectral analyses and chemical correlations with napelline ( 3) and lucidusculine ( 4), respectively.


