Buddleja officinalis
Buddleja officinalis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
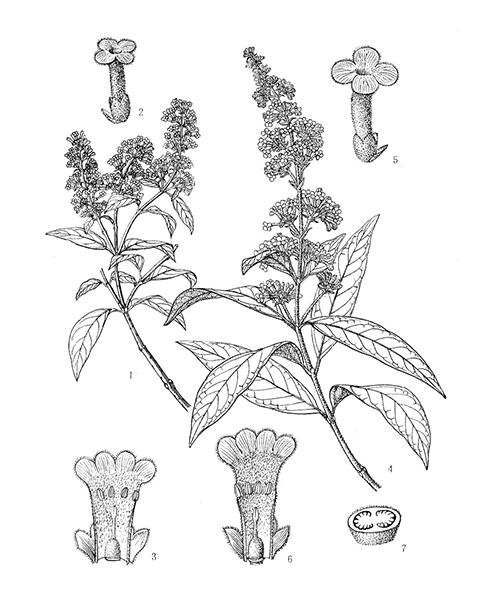
Natural products/compounds from Buddleja officinalis
- Cat.No. Product Name CAS Number COA
-
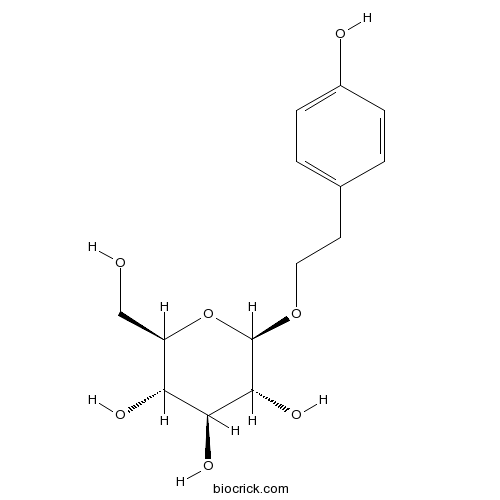
BCN5966
Salidroside10338-51-9
Instructions
-
BCN6059
Syringin118-34-3
Instructions

-
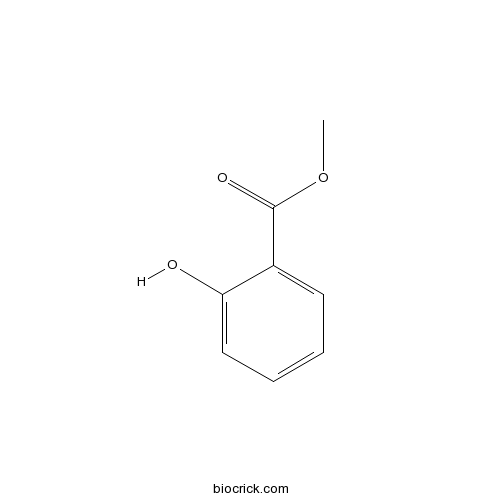
BCN5372
Methyl salicylate119-36-8
Instructions
-
BCN5554
Linarin480-36-4
Instructions

-
BCN5658
Apigenin520-36-5
Instructions

-
BCN5388
Luteolin-7-O-glucoside5373-11-5
Instructions

-
BCN5788
Cosmosiin578-74-5
Instructions

-
BCN4136
Acteoside61276-17-3
Instructions

-
BCN4953
Echinacoside82854-37-3
Instructions
-
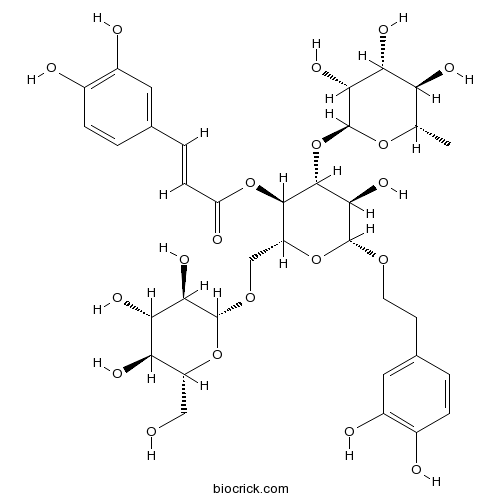
BCN1204
Poliumoside94079-81-9
Instructions

-
BCN5964
Eugenol97-53-0
Instructions
Optimization of the Extraction Conditions for Buddleja officinalis Maxim. Using Response Surface Methodology and Exploration of the Optimum Harvest Time.[Pubmed: 29104270]
None
Identification of the Major Components of Buddleja officinalis Extract and Their Metabolites in Rat Urine by UHPLC-LTQ-Orbitrap.[Pubmed: 27603972]
Buddleja officinalis Maxim, one of the most popular herbal medicines in China, is widely prescribed for curing eye diseases for centuries. In this study, the major components of B. officinalis extract (BOE) and their metabolites in rat urine were detected and identified by ultra-high-pressure liquid chromatography coupled with linear ion trap-orbitrap tandem mass spectrometry (UHPLC-LTQ-Orbitrap). A total of 19 compounds, including 8 flavonoids and 11 phenylethanoid glycosides, were confirmed or tentatively identified from BOE. In vivo, 33 components, including 3 prototypes and 30 metabolies, were confirmed or tentatively identified in rat urine samples. The metabolic pathways of different types of compounds were also proposed. This study would effectively narrow the range of potentially bioactive constituents of BOE and shed light to its action mechanism.
Genetic and Epigenetic Approaches for the Possible Detection of Adulteration and Auto-Adulteration in Saffron (Crocus sativus L.) Spice.[Pubmed: 26978342]
Saffron (Crocus sativus L.) is very expensive and, because of this, often subject to adulteration. Modern genetic fingerprinting techniques are an alternative low cost technology to the existing chemical techniques, which are used to control the purity of food products. Buddleja officinalis Maxim, Gardenia jasminoides Ellis, Curcuma longa L., Carthamus tinctorius L. and Calendula officinalis L. are among the most frequently-used adulterants in saffron spice. Three commercial kits were compared concerning the ability to recover PCR-grade DNA from saffron, truly adulterated samples and possible adulterants, with a clear difference among them, mainly with the processed samples. Only one of the three kits was able to obtain amplifiable DNA from almost all of the samples, with the exception of extracts. On the recovered DNA, new markers were developed based on the sequence of the plastid genes matK and rbcL. These primers, mainly those developed on matK, were able to recognize saffron and the adulterant species and also in mixtures with very low percentages of adulterant. Finally, considering that the addition of different parts of saffron flowers is one of the most widespread adulterations, by analyzing the DNA of the different parts of the flower (styles, stamens and tepals) at the genetic and epigenetic level, we succeeded in finding differences between the three tissues that can be further evaluated for a possible detection of the kind of fraud.
Linarin Inhibits the Acetylcholinesterase Activity In-vitro and Ex-vivo.[Pubmed: 26330885]
Linarin is a flavone glycoside in the plants Flos chrysanthemi indici, Buddleja officinalis, Cirsium setosum, Mentha arvensis and Buddleja davidii, and has been reported to possess analgesic, antipyretic, anti-inflammatory and neuroprotective activities. In this paper, linarin was investigated for its AChE inhibitory potential both in-vitro and ex-vivo. Ellman's colorimetric method was used for the determination of AChE inhibitory activity in mouse brain. In-vitro assays revealed that linarin inhibited AChE activity with an IC50 of 3.801 ± 1.149 μM. Ex-vivo study showed that the AChE activity was significantly reduced in both the cortex and hippocampus of mice treated intraperitoneally with various doses of linarin (35, 70 and 140 mg/Kg). The inhibition effects produced by high dose of linarin were the same as that obtained after huperzine A treatment (0.5 mg/Kg). Molecular docking study revealed that both 4'-methoxyl group and 7-O-sugar moiety of linarin played important roles in ligand-receptor binding and thus they are mainly responsible for AChE inhibitory activity. In view of its potent AChE inhibitory activity, linarin may be a promising therapeutic agent for the treatment of some diseases associated with AChE, such as glaucoma, myasthenia gravis, gastric motility and Alzheimer's disease.
Inhibition of lipopolysaccharide-induced proinflammatory responses by Buddleja officinalis extract in BV-2 microglial cells via negative regulation of NF-kB and ERK1/2 signaling.[Pubmed: 23912273]
Buddleja officinalis has been traditionally used in the supportive treatment of inflammatory and neuronal diseases in Korea and China. Although several reports have shown the anti-inflammatory effects of Buddleja officinalis, the anti-neuroinflammatory effect has remained unclear. In this study, we aimed to investigate the inhibitory effects of flower buds of B. officinalis Maximowicz water extract (BOWE) on LPS-induced inflammatory processes in BV-2 microglial cells. BOWE dose-dependently inhibited the production of nitric oxide as well as iNOS mRNA expression. Moreover, BOWE prevented IL-1β and IL-6 mRNA expression. However, BOWE had no effect on LPS-induced COX-2 or TNF-a mRNA expression. The extract also had no effect on LPS-stimulated p38 MAPK, JNK, and c-Jun phosphorylation, whereas ERK1/2 phosphorylation was strongly inhibited by BOWE. BOWE also inhibited the LPS-induced degradation of IkB-α, and LPS-induced phosphorylation of p65 NF-kB protein. These data indicate that BOWE inhibited the nitric oxide production and pro-inflammatory gene expression in BV-2 microglial cells, possibly through a negative regulation of the NF-kB and ERK1/2 pathways. Further identification of the direct target molecule(s) of BOWE is required to support its use as an anti-neuroinflammatory agent against the neurodegenerative disorders.


