Callicarpa kwangtungensis
Callicarpa kwangtungensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Callicarpa kwangtungensis
- Cat.No. Product Name CAS Number COA
-
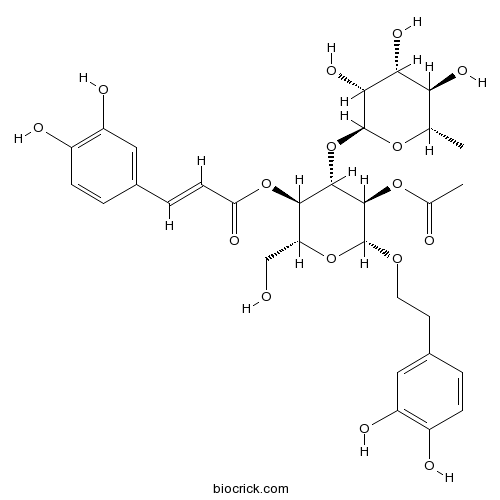
BCN6770
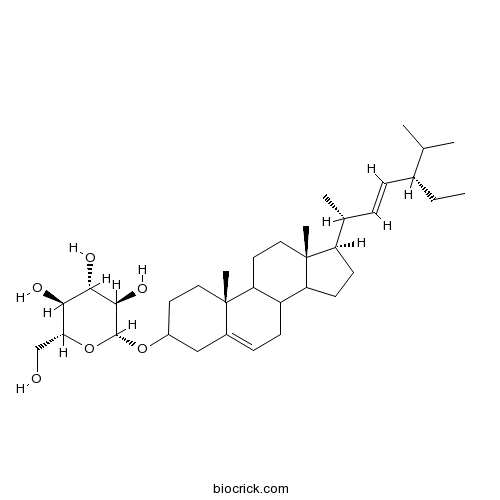
Brandioside133393-81-4
Instructions

-
BCN4865
Stigmasterol glucoside19716-26-8
Instructions
-
BCN5524
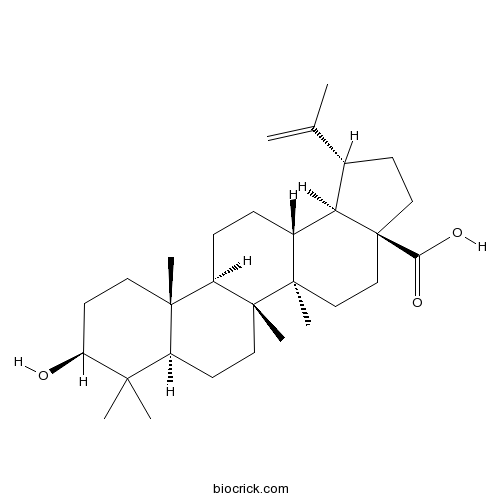
Betulinic acid472-15-1
Instructions
-
BCN5531
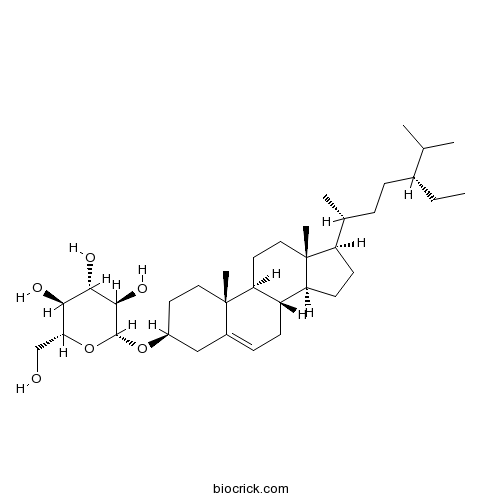
Daucosterol474-58-8
Instructions
-
BCN5699
Syringic acid530-57-4
Instructions

-
BCC4109
Salicylic acid69-72-7
Instructions

-
BCN1205
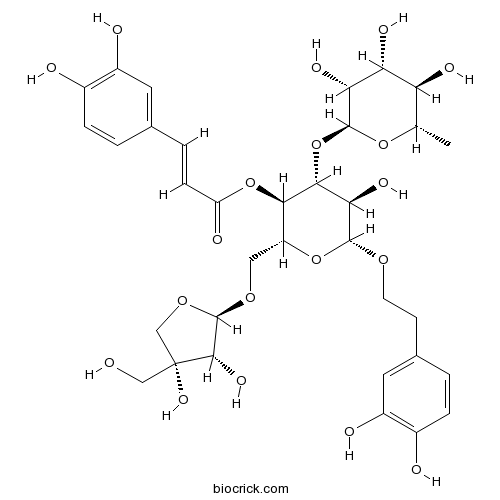
Forsythoside B81525-13-5
Instructions
-
BCN1204
Poliumoside94079-81-9
Instructions

-
BCN3409
2'-Acetylacteoside94492-24-7
Instructions
-
BCN6433
Kajiichigoside F195298-47-8
Instructions

Natural NO inhibitors from the leaves of Callicarpa kwangtungensis: Structures, activities, and interactions with iNOS.[Pubmed: 27989668]
A phytochemical investigation to obtain new NO inhibitors resulted in the isolation of a new diterpenoid with a rare 9,10-seco-abietane skeleton (1) and twelve known terpenoids (2-13) from Callicarpa kwangtungensis. Their structures were elucidated on the basis of extensive 1D and 2D NMR spectroscopic data analyses, and the absolute configuration of compound 1 was established by comparison of the calculated and experimental electronic circular dichroism (ECD) spectra. The inhibitory activities on lipopolysaccharide-induced NO production in murine microglial BV-2 cells of these terpenoids were evaluated, and all of the compounds showed inhibitory effects. The following molecular docking studies showed interactions of the bioactive compounds with the iNOS protein.
Diterpenoids from Callicarpa kwangtungensis and their NO inhibitory effects.[Pubmed: 27491749]
A phytochemical investigation of the leaves of Callicarpa kwangtungensis led to the isolation of three new diterpenoids (1-3), callipenes A-C, and eleven known analogues (4-14). Their structures were established on the basis of extensive analysis of NMR spectroscopic data, X-ray diffraction data, and experimental and calculated electronic circular dichroism spectra. Compounds 1 and 2 are rare abietane diterpenoids possessing a peroxide bridge. All of the isolates were found to inhibit LPS-induced NO production in BV-2 cells.
[Ethyl Acetate-Soluble Chemical Constituents from Callicarpa kwangtungensis (II)].[Pubmed: 27356382]
To study the ethyl acetate-soluble chemical constituents of Callicarpa kwangtungensis.
[Ethyl acetate-soluble chemical constituents from Callicarpa kwangtungensis].[Pubmed: 26027121]
To study the ethyl acetate-soluble chemical constituents of Callicarpa kwangtungensis.
Four New Triterpenoids from Callicarpa kwangtungensis.[Pubmed: 25996212]
Four new triterpenoids which were identifed as 2α,3β,6β,19α-tetrahydroxy- oleanolic acid 28-O-β-D-glucopyranoside (1), 2-O-β-D-glucopyranosyloxy-3α,19α-di-hydroxyoleanolic acid (2), 2-O-β-D-glucopyranosyloxy-3α,19α-dihydroxyursolic acid (3), 2α,3α,6β,19α-tetrahydroxyursolic acid 28-O-β-D-glucopyranoside (4), were isolated from the aerial parts of Callicarpa kwangtungensis together with three known triterpenoids identified as 2α,3β,21β-trihydroxyursolic acid 28-O-β-D-glucopyranoside (5), 2α,3α,19α,23-tetrahydroxyoleanolic acid 28-O-β-D-glucopyranoside (6), 2α,3α,19α,23-tetrahydroxyursolic acid 28-O-β-D-glucopyranoside (7). Their structures were elucidated by the combination of mass spectrometry (MS), one and two-dimensional NMR experiments.
Two new triterpenoids from Callicarpa kwangtungensis.[Pubmed: 25358254]
Two new triterpenoids, 2α,3β,16α,19α,23-pentahydroxyolean-12-en-28-oic acid (1) and 2α,3α,11α,21α,23-pentahydroxyurs-12-en-28-oic acid (2), were isolated from the aerial parts of Callicarpa kwangtungensis. Their structures were elucidated by 1D and 2D analyses, as well as MS and IR spectra.
[Caffeoyl phenylethanoid glycosides from Callicarpa kwangtungensis].[Pubmed: 25095374]
Phytochemical investigation on the EtOH extract from the aerial part of Callicarpa kwangtungensis led to the isolation and characterization of 10 caffeoyl phenylethanoid glycosides, 2'-acetylacteoside (1), tubuloside E (2), acteoside (3), tubuloside B (4), isoacteoside (5), alyssonoside (6), 2'-acetylforsythoside B (7), brandioside (8), forsythoside B (9), and poliumoside (10). Compound 4 was isolated from the plants of Verbenaceae,and 6 was obtained from the Callicarpa genus, for the first time, while compounds 1, 2, 5 and 7 were firstly reported from the plant.
Isolation, identification and activities of natural antioxidants from Callicarpa kwangtungensis Chun.[Pubmed: 24667350]
Reactive oxygen species leads to some diseases associated with oxidative stress. Callicarpa kwangtungensis Chun (CK) is a common remedy in traditional Chinese medicine and possesses diverse biological activities involving antioxidant properties; its main compounds phenylethanoid glycosides (PG) and flavonoids are always reported as antioxidants. In order to develop CK as a safe and activated antioxidant, our investigation was performed to validate antioxidant properties and assess which types of compounds (similar polarity or similar structure), even which compounds, played the role of antioxidants. The extracted compounds of CK were analyzed qualitatively and quantitatively by HPLC-DAD-ESI-Trap MS and UV for their contents and antioxidant activities. The correlations between antioxidant activities and known contents were respectively counted and a semi-quantitative experiment was designed to screen antioxidant compounds of CK with HPLC-UV. The n-butanol fraction (BF) showed the highest total phenolic and flavonoid contents (TPC, TFC), and three PG (forsythiaside B, poliumoside and acteoside) contents. BF showed the significantly best (P<0.05) activities in most assays. There were significant correlations (P<0.05) between DPPH•, ABTS(+)•, •O2(-) scavenging, Cu(2+)-chelating, anti-lipidperoxidation activities and TPC. BF also has significant antioxidant activities on CCl4-induced acute liver injury Mice and TBHP-reduced HepG2 cells. Nine PG (forsythiaside B, poliumoside, acteoside, alyssonoside, brandioside and their derivatives) and one flavone (rhamnazin) were screened out as antioxidants. BF in CK contained abundant polyphenolic, which reflected some definite antioxidant properties. The antioxidant compounds consisted at the least of nine PG and one flavone.
[Determination of forsythiaside B and poliumoside in different origin and parts from Callicarpa kwangtungensis].[Pubmed: 24422401]
The RP-HPLC method was used to determinate the contents of forsythiaside B and poliumoside in different origin and parts from Callicarpa kwangtungensis. The linear ranges of forsythiaside B and poliumoside were 0. 106-3. 18 and 0. 105 2-3. 156 microg, respectively. The average recoveries of forythiaside B and poliumoside were 99. 01% ( RSD 1. 2%) and 100. 13% (RSD 0. 90% ), respectively. The contents of forsythiaside B and poliumoside were changed in different origin and parts from C. kwangtungensis. The sample from the area of Luxi, Pingxiang City, Jiangxi Province has the highest contents of forsythiaside B and poliumoside. The contents of forsythiaside B and poliumoside in different parts from C. kwangtungensis in Luxi are: leaf > stem > fruit. This result will provide a scientific basis for quality control and reasonable utilzation of C. kwangtungensis.


