Callistemon viminalis
Callistemon viminalis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Callistemon viminalis
- Cat.No. Product Name CAS Number COA
-
BCN3823
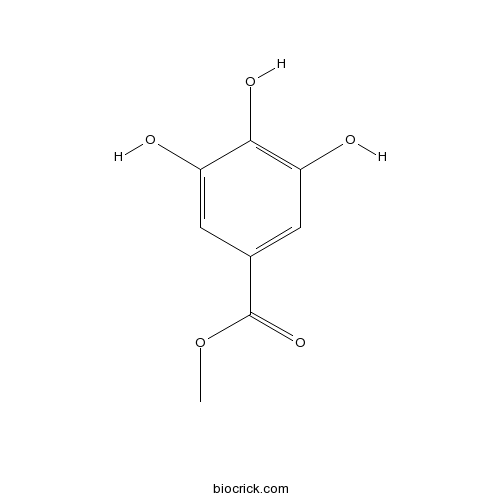
Methyl gallate99-24-1
Instructions
Two new 12-membered macrolides from the endophytic fungal strain Cladosprium colocasiae A801 of Callistemon viminalis.[Pubmed: 29741104]
Two new polyketide metabolites, the 12-membered macrolides 4-hydroxy-12-methyloxacyclododecane-2,5,6-trione (1) and 12-methyloxacyclododecane-2,5,6-trione (2), were isolated from the endophytic fungal strain Cladosprium colocasiae A801 of the plant Callistemon viminalis, together with five known derivatives. Their structures were fully characterized by means of detailed spectroscopic analysis for new structures, and in comparison with published data for known compounds. The antibacterial, cytotoxic, and α-glucosidase inhibitory activities of the new compounds 1 and 2 were evaluated.
Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): their physical properties and novel biological applications.[Pubmed: 29400584]
None
Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria.[Pubmed: 29126952]
Three bacterial isolates were isolated from infected potato tubers showing soft and brown rots like symptoms as well as one isolate from infected peach tree showing crown gall symptom. The morphological, biochemical and molecular assays proved that bacterial isolates belonging to Pectobacterium carotovorum subsp. carotovorum, Ralstonia solanacearum, Dickeya spp. and Agrobacterium tumefaciens. The acetone (AcE) and n-butanol (ButE) extracts of Callistemon viminalis flowers and essential oil from aerial parts of Conyza dioscoridis as well as ButE of Eucalyptus camaldulensis bark are evaluated at different concentrations against the growth of the isolated bacteria. The diameter of inhibition zone (IZ) and the minimum inhibitory concentrations (MICs) are compared. Results indicated that the highest IZ values were 20.0 mm and 18.3 mm for E. camaldulensis bark ButE and C. viminalis flower ButE, respectively, against P. carotovorum; 16.3 mm and 16.0 mm for E. camaldulensis bark ButE and C. viminalis flower ButE, respectively, against R. solanacearum; 18.5 mm for C. viminalis flower AcE and C. dioscoridis aerial parts EO against Dickeya spp.; and 15.0 mm for C. viminalis flower AcE against A. tumefaciens. MICs ranged from <16 μg/mL for D. solani to >4000 μg/mL for A. tumefaciens. It was proved that C. viminalis flowers AcE contains mainly 5-hydroxymethylfurfural (20.6%), palmitic acid (18.5%), and pyrogallol (16.4%); while C. viminalis flower ButE contains palmitic acid (36.3%), 2-hydroxymyristic acid (9.4%), 5-hydroxymethylfurfural (7.2%), and shikimic acid (6.6%); whereas E. camaldulensis bark ButE contains 8-nonynoic acid methyl ester (45.6), camphor (30.9%), menthol (8.8%), and 1,8-cineole (eucalyptol) (8.2%), whilst the EO of C. dioscoridis aerial parts comprises Z-(13,14-epoxy)tetradec-11-en-1-ol acetate (11.6%), γ-elemene (10.2%), tau.-muurolol (7.1%), and cadina-3,9-diene (4.7%). It can be concluded that phytochemical extracts of C. viminalis, E. camaldulensis and C. dioscoridis demonstrated strong to moderate antibacterial effects against the studied plant bacterial pathogens.
Callistemenonone A, a novel dearomatic dibenzofuran-type acylphloroglucinol with antimicrobial activity from Callistemon viminalis.[Pubmed: 28539599]
A new acylphloroglucinol with a novel architecture including an unprecedented dearomatic dibenzofuran core, named callistemenonone A (1), was isolated from the leaves of Callistemon viminalis (Myrtaceae). The structure was fully characterized on the basis of extensive spectroscopic analysis, including UV, HRESIMS, as well as 1D and 2D NMR spectral data (HSQC, HMBC, and ROESY). The deduced structure represents the first example of a natural dibenzofuran with two phenyl moieties coupling through tertiary hydroxy and ketal carbons. A plausible biogenetic pathway involving oxidative coupling and dearomatization as key steps is proposed to account for the biosynthesis of this novel class of dibenzofuran. Moreover, antimicrobial assays, in conjunction with the time-killing and biophysical studies, revealed that 1 exerted potent bactericidal activity against a panel of methicillin resistant pathogenic microbes with a unique mechanism.
Medicinal and biological values of Callistemon viminalis extracts: History, current situation and prospects.[Pubmed: 28442106]
Callistemon viminalis (C. viminalis) is a plant that has been reported to have various medicinal values such as antibacterial, antifungal, antioxidant activities and other pharmaceutical and insecticidal properties. This review covers the potentials, applications and properties of different extracts from different parts (branches, flowers, fruits, bark, leaves) of C. viminalis. Furthermore, the chemical structures of the bioactive compounds were reported for biological activities. All the results supported the traditional uses of C. viminalis in folk medicine. In addition, some researches supported the use of C. viminalis extracts for the preparation of metal oxide nanoparticles.
Callviminols A-E, new terpenoid-conjugated phloroglucinols from the leaves of Callistemon viminalis.[Pubmed: 27777133]
Callviminols A-E (1-5), five rare phloroglucinols bearing a framework embodying a hexahydrodibenzo[b,d]furan or 2-phenylcyclohexanol nucleus derived from a phloroglucinol-monoterpene adduct, were isolated from the leaves of Callistemon viminalis. Their structures were established via extensive spectroscopic measurements, with the absolute configuration of 5 determined by electronic circular dichroism (ECD) calculations. The plausible biogenetic pathway suggested that a unique oxidative radical addition and classic cationic cyclization were key biosynthetic steps.
Callistiviminenes A-O: Diverse adducts of β-triketone and sesqui- or monoterpene from the fruits of Callistemon viminalis.[Pubmed: 27614821]
None
Acylphloroglucinols from the leaves of Callistemon viminalis.[Pubmed: 27565545]
A phytochemical study on the leaves of Callistemon viminalis, a widely distributed ornamental and medicinal plant of agricultural importance in China, resulted in the isolation of eleven acylphloroglucinols, including six new ones named callistenones F-K (1-6), as well as five known congeners. Their structures were fully characterized using spectral data interpretation for the new structures and compared to published data for the known ones. All the isolated compounds were evaluated for in vitro antimicrobial activity and growth inhibitory activity against four tumor cell lines (MCF-7, NCI-H460, SF-268 and HepG-2).
Differential Larval Toxicity and Oviposition Altering Activity of Some Indigenous Plant Extracts against Dengue and Chikungunya Vector Aedes albopictus.[Pubmed: 26114131]
Mosquitoes are well known as vectors of several disease causing pathogens. The extensive use of synthetic insecticides in the mosquito control strategies resulted to the development of pesticide resistance and fostered environmental deterioration. Hence in recent years plants become alternative source of mosquito control agents. The present study assessed the larvicidal and oviposition altering activity of six different plants species-Alstonia scholaris, Callistemon viminalis, Hyptis suaveolens, Malvastrum coromandelianum, Prosopis juliflora, Vernonia cinerea against Aedes albopictus mosquito in laboratory.
Molluscicidal Activity of the Methanol Extract of Callistemon viminalis (Sol. ex Gaertner) G.Don ex Loudon Fruits, Bark and Leaves against Biomphalaria alexandrina Snails.[Pubmed: 25237345]
Methanol extracts of Callistemon viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves were tested for molluscicidal activity. Snails were collected and kept in dechlorinated water under standard condition. Ten adults Biomphalaria Alexandrina, of the same size, were introduced in plastic acquaria for each experiment. The fruits, barks and leaves were extracted with methanol and the methanol extracts were kept for testing as molluscicides. Different extracts proved to have molluscicidal activity against the vector of schistosomiasis, B. alexandrina snails. LC50 values for C. viminalis fruits, bark and leaves were 6.2, 32 and 40 ppm respectively. The C. viminalis fruits extract showed the highest effect against the tested snails. Histopathological studies proved that the site of action of all tested extracts was localized in the digestive system and hermaphrodite gland.


