Methyl gallateCAS# 99-24-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99-24-1 | SDF | Download SDF |
| PubChem ID | 7428 | Appearance | Beige powder |
| Formula | C8H8O5 | M.Wt | 184.15 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Gallic acid methyl ester; Methyl 3,4,5-trihydroxybenzoate; 3,4,5-Trihydroxybenzoic acid methyl ester | ||
| Solubility | DMSO : ≥ 160 mg/mL (868.86 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | methyl 3,4,5-trihydroxybenzoate | ||
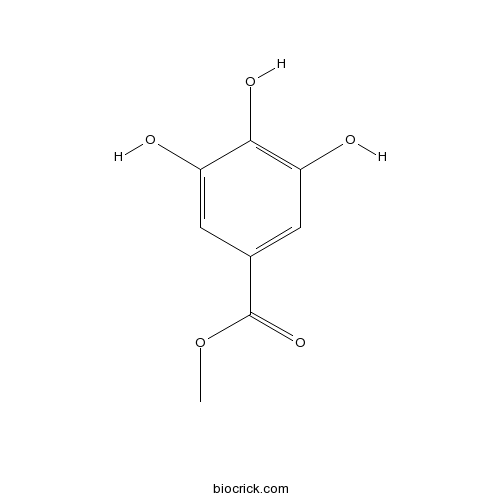
| SMILES | COC(=O)C1=CC(=C(C(=C1)O)O)O | ||
| Standard InChIKey | FBSFWRHWHYMIOG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O5/c1-13-8(12)4-2-5(9)7(11)6(10)3-4/h2-3,9-11H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methyl Gallate is a plant polyphenol with antioxidant, anticancer, anti-bacterial, and anti-inflammatory activities.Methyl gallate is a potent and highly specific inhibitor of herpes simplex virus in vitro. Methyl gallate also has a dual cyclooxygenase-2/5-lipoxygenase inhibitory activity. Methyl gallate can inhibit the growth of oral pathogens and S. mutans biofilm formation, and may be used to prevent the formation of oral biofilms. |
| Targets | COX | PGE | ROS | IL Receptor | gp120/CD4 | CXCR | HSV |
| In vitro | Inhibitory effect of methyl gallate and gallic acid on oral bacteria.[Pubmed: 19107406 ]J Microbiol. 2008 Dec;46(6):744-50.This study examined the ability of Methyl gallate (MG) and gallic acid (GA), the main compounds of gallo-tannins in Galla Rhois, to inhibit the proliferation of oral bacterial and the in vitro formation of Streptococcus mutans biofilms. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria.[Pubmed: 19471197]Molecules. 2009 May 11;14(5):1773-80.Methyl gallate is a major component of Galla Rhois, as carvacrol is of oregano essential oils. Both have shown good antibacterial activity against intestinal bacteria. |
| In vivo | Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells.[Pubmed: 21048105]J Immunol. 2010 Dec 1;185(11):6698-705.CD4(+)CD25(+) regulatory T (Treg) cells play crucial roles in the host response to tumors. Increasing evidence supports the existence of elevated numbers of Treg cells in solid tumors and hematologic malignancies. |
| Kinase Assay | Effects of methyl gallate on arachidonic acid metabolizing enzymes: Cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells.[Pubmed: 17121182]Arch Pharm Res. 2006 Oct;29(10):874-8.
|
| Cell Research | Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells.[Pubmed: 15046831]Food Chem Toxicol. 2004 May;42(5):843-50.Methyl gallate (MG) has been shown to be an effective antioxidant in a variety of acellular experiments. |
| Structure Identification | Biosci Rep. 1988 Feb;8(1):95-102.Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives.[Pubmed: 2840133]
|

Methyl gallate Dilution Calculator

Methyl gallate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4304 mL | 27.1518 mL | 54.3036 mL | 108.6071 mL | 135.7589 mL |
| 5 mM | 1.0861 mL | 5.4304 mL | 10.8607 mL | 21.7214 mL | 27.1518 mL |
| 10 mM | 0.543 mL | 2.7152 mL | 5.4304 mL | 10.8607 mL | 13.5759 mL |
| 50 mM | 0.1086 mL | 0.543 mL | 1.0861 mL | 2.1721 mL | 2.7152 mL |
| 100 mM | 0.0543 mL | 0.2715 mL | 0.543 mL | 1.0861 mL | 1.3576 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Trehalose
Catalog No.:BCC9182
CAS No.:99-20-7
- Prunasin
Catalog No.:BCN4535
CAS No.:99-18-3
- Ac-DL-Leu-OH
Catalog No.:BCC2977
CAS No.:99-15-0
- 3,5-DHBA
Catalog No.:BCC7951
CAS No.:99-10-5
- Fmoc-Arg(Mtr)-OH
Catalog No.:BCC3074
CAS No.:98930-01-9
- Fmoc-His(Fmoc)-OH
Catalog No.:BCC3500
CAS No.:98929-98-7
- Limonol
Catalog No.:BCN4533
CAS No.:989-61-7
- (-)-Epigallocatechin gallate
Catalog No.:BCN6326
CAS No.:989-51-5
- 3-Epiursolic acid
Catalog No.:BCN3173
CAS No.:989-30-0
- Pseudolaric acid A-O-beta-D-glucopyranoside
Catalog No.:BCN1290
CAS No.:98891-44-2
- Pseudolaric acid B-O-beta-D-glucopyranoside
Catalog No.:BCN1291
CAS No.:98891-41-9
- Danshenxinkun D
Catalog No.:BCN2472
CAS No.:98873-76-8
- Chelidonic acid
Catalog No.:BCN6547
CAS No.:99-32-1
- 2-Methyl-5-Isopropenyl-2-Cyclohexenone
Catalog No.:BCC8279
CAS No.:99-49-0
- 3,4-Dihydroxybenzoic acid
Catalog No.:BCN4537
CAS No.:99-50-3
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
- Methyl 4-hydroxybenzoate
Catalog No.:BCN4540
CAS No.:99-76-3
- 4-Isopropyltoluene
Catalog No.:BCC8282
CAS No.:99-87-6
- 4'-Hydroxyacetophenone
Catalog No.:BCN4544
CAS No.:99-93-4
- 4-Hydroxybenzoic acid
Catalog No.:BCN4546
CAS No.:99-96-7
- Fentanyl citrate
Catalog No.:BCC6000
CAS No.:990-73-8
- Imiquimod
Catalog No.:BCC2492
CAS No.:99011-02-6
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
- [Ala107]-MBP (104-118)
Catalog No.:BCC5835
CAS No.:99026-77-4
Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells.[Pubmed:15046831]
Food Chem Toxicol. 2004 May;42(5):843-50.
Methyl gallate (MG) has been shown to be an effective antioxidant in a variety of acellular experiments. Accordingly, this study was designed to assess the ability of MG, extracting from Toona sinensis to protect cultured Madin-Darby canine kidney (MDCK) cells against hydrogen peroxide (H2O2)-mediated oxidative stress. Trolox, a cell permeable and water-soluble vitamin E analogue, was included for comparison. First, when MDCK cells were pretreated with MG and trolox for 1 h, followed by exposing to H2O2 (0.8 mM) for an additional hour, we found that the intracellular peroxide productions, as reflected by dichlorofluorescein (DCF) fluorescence, were shown to be decreased in a concentration-dependent manner. Furthermore, using C11-BODIPY581/591 as a lipid peroxidation probe, we also found that MG, in a concentration of 100 microM, could alleviate lipid peroxidation of the cells exposed to a short-term H2O2 treatment. In addition, MG-treated cells could prevent intracellular glutathione (GSH) from being depleted following an exposure of H2O2 (8.0 mM) for a 3 h period. Next, we also examined the effect of MG on H2O2-mediated oxidative damage to DNA. Using 8-oxoguanine as an indicator for oxidative DNA damage, we demonstrated that the percentage of MDCK cells containing 8-oxoguanine was drastically increased by exposing to H2O2 (40 mM) for 3 h. However, 8-oxoguanine contents were shown to be significantly decreased in the presence of MG prior to H2O2 exposure. Comparatively, MG was shown to be a better protective agent against oxidative damage to DNA as compared to trolox. Taken together, our data suggest that MG is effective in preventing H2O2-induced oxidative stress and DNA damage in MDCK cells. The underlying mechanisms involved scavenging of intracellular reactive oxygen species (ROS), inhibition of lipid peroxidation and prevention of intracellular GSH depletion.
Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives.[Pubmed:2840133]
Biosci Rep. 1988 Feb;8(1):95-102.
Methyl gallate (MG), methyl-3,4,5-trihydroxybenzoate, was highly active against herpes viruses as determined by plaque reduction assay. Herpes simplex virus type 2, MS strain, was sensitive to MG at a mean 50% inhibitory concentration (IC50) of 0.224 micrograms/ml in monkey kidney cells. MG was specific for herpes viruses with the relative sensitivity HSV-2 greater than HSV-1 greater than CMV. Two RNA viruses tested were significantly less sensitive to MG. The structural components of MG which modulate the anti-herpetic activity were identified by analysis of chemical analogues. Our structural analyses indicated that three hydroxyl groups were required but were not sufficient for the anti-herpetic action of MG. The presence and chain length of the alkyl ester were also important to the anti-herpetic activity of MG. Methyl gallate may interact with virus proteins and alter the adsorption and penetration of the virion.
Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells.[Pubmed:21048105]
J Immunol. 2010 Dec 1;185(11):6698-705.
CD4(+)CD25(+) regulatory T (Treg) cells play crucial roles in the host response to tumors. Increasing evidence supports the existence of elevated numbers of Treg cells in solid tumors and hematologic malignancies. In this study, the effects of Methyl gallate on Treg cells were examined. Methyl gallate inhibited Treg cell-suppressive effects on effector CD4(+) T cells and Treg migration toward tumor environment. The expression of Treg surface markers including CTLA-4, CCR4, CXCR4, and glucocorticoid-induced TNFR was significantly suppressed upon Methyl gallate treatment. Furthermore, forkhead box P3 (Foxp3) expression was also significantly decreased by Methyl gallate, suggesting that the suppressive effects of Methyl gallate on Treg were medicated by decrease of Treg-specific transcription factor Foxp3. In tumor-bearing hosts, Methyl gallate treatment substantially reduced tumor growth and prolonged the survival rate. In contrast, nu/nu mice did not show decreased tumor progression in response to Methyl gallate. In addition, in tumor-bearing Treg-depleted mice, tumor growth and the survival rates were not changed by Methyl gallate treatment, strongly suggesting that the main therapeutic target of Methyl gallate in tumor suppression was related to modulation of the CD4(+)CD25(+) Treg cell functions. In the spleen of tumor-bearing mice, Methyl gallate treatment induced a significant decrease in the CD4(+)CD25(+)Foxp3(high) Treg cell population. Especially, the number of tumor-infiltrating CD25(+)Foxp3(high) Treg cells was significantly lower in Methyl gallate-treated mice. These results suggest that Methyl gallate can be used to reverse immune suppression and as a potentially useful adjunct for enhancing the efficacy of immune-based cancer therapy.
Inhibitory effect of methyl gallate and gallic acid on oral bacteria.[Pubmed:19107406]
J Microbiol. 2008 Dec;46(6):744-50.
This study examined the ability of Methyl gallate (MG) and gallic acid (GA), the main compounds of gallo-tannins in Galla Rhois, to inhibit the proliferation of oral bacterial and the in vitro formation of Streptococcus mutans biofilms. The antimicrobial activities of these compounds were evaluated in vitro using the broth microdilution method and a beaker-wire test. Both MG and GA had inhibitory effects on the growth of cariogenic (MIC<8 mg/ml) and periodontopathic bacteria (MIC=1 mg/ml). Moreover, these compounds significantly inhibited the in vitro formation of S. mutans biofilms (MG, 1 mg/ml; GA, 4 mg/ml; P<0.05). MG was more effective in inhibiting bacterial growth and the formation of S. mutans biofilm than GA. In conclusion, MG and GA can inhibit the growth of oral pathogens and S. mutans biofilm formation, and may be used to prevent the formation of oral biofilms.
Effects of methyl gallate on arachidonic acid metabolizing enzymes: Cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells.[Pubmed:17121182]
Arch Pharm Res. 2006 Oct;29(10):874-8.
Methyl gallate (MG) is a medicinal herbal product that is isolated from Paeonia lactiflora that inhibits cyclooxygenase-2 (COX-2) dependent phases of prostaglandin D2 (PGD2) generation in bone marrow-derived mast cells (BMMC) in a concentration-dependent manner with an IC50 values of 17.0 microM. This compound also found inhibited the COX-2-dependent conversion of the exogenous arachidonic acid to PGD2 in a dose-dependent manner with an IC50 values of 19.0 microM, using a COX enzyme assay kit. However, at concentrations up to 80 microM, MG did not inhibit COX-2 protein expression in BMMC, indicating that MG inhibits COX-2 activity directly. Furthermore, MG consistently inhibited the production of leukotriene C4 (LTC4) in a dose dependent manner, with an IC50 value of 5.3 microM. These results demonstrate that MG has a dual cyclooxygenase-2/5-lipoxygenase inhibitory activity, which might provide the basis for novel anti-inflammatory drugs.
Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria.[Pubmed:19471197]
Molecules. 2009 May 11;14(5):1773-80.
Methyl gallate is a major component of Galla Rhois, as carvacrol is of oregano essential oils. Both have shown good antibacterial activity against intestinal bacteria. This study investigated the antibacterial activities of nalidixic acid in combination with Methyl gallate and carvacrol against nalidixic acid resistant bacteria. The combined effect of nalidixic acid with Methyl gallate and carvacrol was evaluated using the checkerboard method to obtain a fractional inhibitory concentration index. The results showed that the combinations of nalidixic acid + Methyl gallate/carvacrol improved nalidixic acid resistant pathogenic bacteria inhibition with synergy or partial synergy activity. Thus, a strong bactericidal effect of the drug combinations was observed. In vitro data thus suggested that nalidixic acid combined with Methyl gallate and carvacrol may be microbiologically beneficial, rather than antagonists.


