3,4-Dihydroxybenzoic acidCAS# 99-50-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 99-50-3 | SDF | Download SDF |
| PubChem ID | 72 | Appearance | White-beige powder |
| Formula | C7H6O4 | M.Wt | 154.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | 3,4-Dihydroxybenzoic acid | ||
| Solubility | DMSO : 50 mg/mL (324.42 mM; Need ultrasonic) H2O : 10 mg/mL (64.88 mM; Need ultrasonic) | ||
| Chemical Name | 3,4-dihydroxybenzoic acid | ||
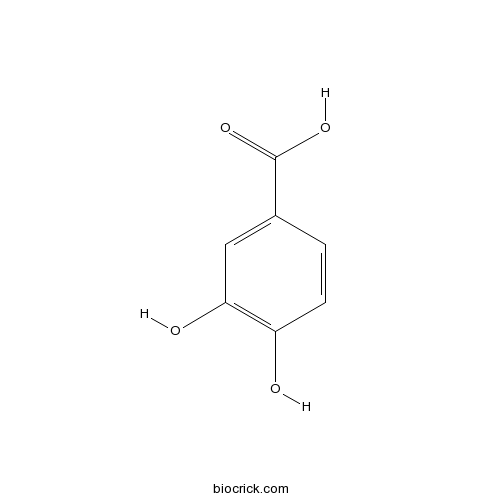
| SMILES | C1=CC(=C(C=C1C(=O)O)O)O | ||
| Standard InChIKey | YQUVCSBJEUQKSH-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,4-Dihydroxybenzoic acid shows significant nematicidal, antitumor, neuroprotective, antioxidant, and antimicrobial activities. 3,4-Dihydroxybenzoic acid has protection against adriamycin cytotoxicity and inhibition of DNA topoisomerase II activity; it also protects human keratinocytes against UVB-induced oxidative stress and apoptosis.3,4-Dihydroxybenzoic acid can prevent Abeta (25-35)-induced neuronal cell damage by interfering with the increase of [Ca(2+)](c), and then by inhibiting glutamate release, generation of ROS and caspase-3 activity. |
| Targets | Beta Amyloid | Calcium Channel | ROS | Caspase | JNK | p38MAPK | p53 | Topoisomerase | Bcl-2/Bax | Antifection |
| In vitro | Protective effect of 3,4-dihydroxybenzoic acid isolated from Cladophora wrightiana Harvey against ultraviolet B radiation-induced cell damage in human HaCaT keratinocytes.[Pubmed: 24414942]Appl Biochem Biotechnol. 2014 Mar;172(5):2582-92.The aim of the present study was to elucidate the protective properties of 3,4-Dihydroxybenzoic acid (DBA) isolated from Cladophora wrightiana Harvey (a green alga) against ultraviolet B (UVB)-induced damage to human HaCaT keratinocytes. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita.[Pubmed: 23603737]Microb Pathog. 2013 Jun-Jul;59-60:52-9.In this study, the 3,4-Dihydroxybenzoic acid (3,4-DHBA) from Terminalia nigrovenulosa bark (TNB) was purified and its in vitro nematicidal activity was investigated against Meloidogyne incognita. 3,4-dihydroxybenzoic acid and 3,4-dihydroxybenzaldehyde from the fern Trichomanes chinense L.; isolation, antimicrobial and antioxidant properties.[Reference: WebLink]Indonesian Journal of Chemistry, 2012, 12(3):273-8.3,4-Dihydroxybenzoic acid (1) and 3,4-dihydroxybenzaldehyde (2) have been isolated from ethyl acetate fraction of methanolic fractions of leaves, stems and roots of the fern Trichomanes chinense L. (Hymenophyllaceae).
|
| Kinase Assay | Protection against Adriamycin cytotoxicity and inhibition of DNA topoisomerase II activity by 3,4-dihydroxybenzoic acid.[Pubmed: 12792789]Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation.[Pubmed: 17304508 ]Int J Cancer. 2007 Jun 1;120(11):2306-16.3,4-Dihydroxybenzoic acid (protocatechuic acid, PCA) is discussed to represent antioxidative food components in a human diet rich in fruits and vegetables, and has been shown to prevent carcinogenesis or antitumor growth in vivo. However, the molecular mechanisms involved in chemopreventive activity of PCA are poorly understood.
Int J Oncol. 2003 Jul;23(1):159-63.The mechanism of Adriamycin (ADR) induced cytotoxicity is not completely understood. While a variety of mechanisms have been proposed, the production of free radicals by redox cycling of the semiquinone has been implicated in cytotoxicity, specifically for cardiotoxicity. |
| Cell Research | 3,4-dihydroxybenzoic acid from Smilacis chinae rhizome protects amyloid beta protein (25-35)-induced neurotoxicity in cultured rat cortical neurons.[Pubmed: 17531386 ]Neurosci Lett. 2007 Jun 13;420(2):184-8.The neuroprotective effect of 3,4-Dihydroxybenzoic acid (3,4-DHBA) isolated from Smilacis chinae rhizome against Abeta (25-35)-induced neurotoxicity on cultured rat cortical neurons was found in this study. |

3,4-Dihydroxybenzoic acid Dilution Calculator

3,4-Dihydroxybenzoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4893 mL | 32.4465 mL | 64.8929 mL | 129.7859 mL | 162.2323 mL |
| 5 mM | 1.2979 mL | 6.4893 mL | 12.9786 mL | 25.9572 mL | 32.4465 mL |
| 10 mM | 0.6489 mL | 3.2446 mL | 6.4893 mL | 12.9786 mL | 16.2232 mL |
| 50 mM | 0.1298 mL | 0.6489 mL | 1.2979 mL | 2.5957 mL | 3.2446 mL |
| 100 mM | 0.0649 mL | 0.3245 mL | 0.6489 mL | 1.2979 mL | 1.6223 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Protocatechuic acid is a phenolic compound which exhibits neuroprotective effect.
In Vitro:Protocatechuic acid inhibits the aggregation of Aβ and αS and destabilizes their preformed fibrils. Protocatechuic acid prevents the death of PC12 cells triggered by Aβ- and αS-induced toxicity[3].
In Vivo:Protocatechuic acid is able to prevent stress induced immobility time in forced swim test without altering locomotor activity in mice. Further, Protocatechuic acid treatment attenuates the elevation of serum corticosterone, lipid peroxidation and restores enzymatic antioxidants in cerebral cortex and hippocampus in ARS mice[1]. Rat administered cadmium and treated with prostigmine and doses of Protocatechuic acid (10−20 mg/kg) has significantly reduced BChE activity. Cadmium and either prostigmine or Protocatechuic acid (10−20 mg/kg) treated rats shows significant reduction in MDA level[2].
References:
[1]. Thakare VN, et al. Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab Brain Dis. 2016 Oct 26.
[2]. Adefegha SA, et al. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed Pharmacother. 2016 Oct;83:559-568.
[3]. Hornedo-Ortega R, et al. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J Agric Food Chem. 2016 Oct 10.
- 2-Methyl-5-Isopropenyl-2-Cyclohexenone
Catalog No.:BCC8279
CAS No.:99-49-0
- Chelidonic acid
Catalog No.:BCN6547
CAS No.:99-32-1
- Methyl gallate
Catalog No.:BCN3823
CAS No.:99-24-1
- Trehalose
Catalog No.:BCC9182
CAS No.:99-20-7
- Prunasin
Catalog No.:BCN4535
CAS No.:99-18-3
- Ac-DL-Leu-OH
Catalog No.:BCC2977
CAS No.:99-15-0
- 3,5-DHBA
Catalog No.:BCC7951
CAS No.:99-10-5
- Fmoc-Arg(Mtr)-OH
Catalog No.:BCC3074
CAS No.:98930-01-9
- Fmoc-His(Fmoc)-OH
Catalog No.:BCC3500
CAS No.:98929-98-7
- Limonol
Catalog No.:BCN4533
CAS No.:989-61-7
- (-)-Epigallocatechin gallate
Catalog No.:BCN6326
CAS No.:989-51-5
- 3-Epiursolic acid
Catalog No.:BCN3173
CAS No.:989-30-0
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
- Methyl 4-hydroxybenzoate
Catalog No.:BCN4540
CAS No.:99-76-3
- 4-Isopropyltoluene
Catalog No.:BCC8282
CAS No.:99-87-6
- 4'-Hydroxyacetophenone
Catalog No.:BCN4544
CAS No.:99-93-4
- 4-Hydroxybenzoic acid
Catalog No.:BCN4546
CAS No.:99-96-7
- Fentanyl citrate
Catalog No.:BCC6000
CAS No.:990-73-8
- Imiquimod
Catalog No.:BCC2492
CAS No.:99011-02-6
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
- [Ala107]-MBP (104-118)
Catalog No.:BCC5835
CAS No.:99026-77-4
- [Ala113]-MBP (104-118)
Catalog No.:BCC5836
CAS No.:99026-78-5
- Limonexic acid
Catalog No.:BCN4534
CAS No.:99026-99-0
- Kushenol I
Catalog No.:BCN2983
CAS No.:99119-69-4
Protective effect of 3,4-dihydroxybenzoic acid isolated from Cladophora wrightiana Harvey against ultraviolet B radiation-induced cell damage in human HaCaT keratinocytes.[Pubmed:24414942]
Appl Biochem Biotechnol. 2014 Mar;172(5):2582-92.
The aim of the present study was to elucidate the protective properties of 3,4-Dihydroxybenzoic acid (DBA) isolated from Cladophora wrightiana Harvey (a green alga) against ultraviolet B (UVB)-induced damage to human HaCaT keratinocytes. DBA exhibited scavenging actions against the 1,1-diphenyl-2-picrylhydrazyl radical, the superoxide anion, and the hydroxyl radical. Furthermore, DBA decreased the levels of intracellular reactive oxygen species generated by hydrogen peroxide or UVB treatment of the cells. DBA also decreased the UVB-augmented levels of phospho-histone H2A.X and the extent of comet tail formation, which are both indications of DNA damage. In addition, the compound safeguarded keratinocytes from UVB-induced injury by reversing the production of apoptotic bodies, overturning the disruption of mitochondrial membrane potential, increasing the expression of the anti-apoptotic protein, B-cell lymphoma 2, and decreasing the expression of the pro-apoptotic proteins, Bcl-2-associated X and cleaved caspase-3. Taken together, these results demonstrate that DBA isolated from a green alga protects human keratinocytes against UVB-induced oxidative stress and apoptosis.
3,4-dihydroxybenzoic acid from Smilacis chinae rhizome protects amyloid beta protein (25-35)-induced neurotoxicity in cultured rat cortical neurons.[Pubmed:17531386]
Neurosci Lett. 2007 Jun 13;420(2):184-8.
The neuroprotective effect of 3,4-Dihydroxybenzoic acid (3,4-DHBA) isolated from Smilacis chinae rhizome against Abeta (25-35)-induced neurotoxicity on cultured rat cortical neurons was found in this study. The protective effect of 3,4-DHBA against Abeta (25-35)-induced neuronal cell death was investigated by measuring cell viability via a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) assay and Hoechst 33342 staining. 3,4-DHBA (1 and 10 microM) concentration-dependently inhibited 10 microM Abeta (25-35)-induced neuronal apoptotic death. 3,4-DHBA (1 and 10 microM) inhibited 10 microM Abeta (25-35)-induced elevation of cytosolic Ca(2+) concentration ([Ca(2+)](c)), which was measured by a fluorescent dye, Fluo-4 AM. 3,4-DHBA also inhibited glutamate release into medium, reactive oxygen species (ROS) generation, and caspase-3 activation, which were induced by 10 microM Abeta (25-35). These results suggest that 3,4-DHBA prevents Abeta (25-35)-induced neuronal cell damage by interfering with the increase of [Ca(2+)](c), and then by inhibiting glutamate release, generation of ROS and caspase-3 activity.
Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation.[Pubmed:17304508]
Int J Cancer. 2007 Jun 1;120(11):2306-16.
3,4-Dihydroxybenzoic acid (protocatechuic acid, PCA) is discussed to represent antioxidative food components in a human diet rich in fruits and vegetables, and has been shown to prevent carcinogenesis or antitumor growth in vivo. However, the molecular mechanisms involved in chemopreventive activity of PCA are poorly understood. In this study, investigations were conducted to examine the detailed signaling pathway involved in PCA-induced apoptosis in human gastric adenocarcinoma (AGS) cells. The data from cell viability assay showed that PCA exhibited the antiproliferation effect on AGS cells in a time- and dose-dependent manner. The occurrence of apoptosis induced by PCA was confirmed by morphological and biochemical features, including apoptotic bodies formation and an increase in the distribution of hypodiploid phase. Molecular data showed the effect of PCA in AGS cells might be mediated via sustained phosphorylation and activation of JNK and p38 mitogen-activating protein kinases (MAPK), but not ERK. Treatment with pharmacological inhibitors or transfection with the mutant p38 or/and JNK expression vector reduced PCA-mediated apoptosis and the JNK/p38 MAPK-related proteins phosphorylation and expression, including ATF-2, c-Jun, FasL, Fas, p53 and Bax. Preincubation with Nok-1 monoclonal antibody, which is inhibitory to Fas signaling, interfered with PCA-induced cleavage of procaspase and sensitization to anti-APO-induced apoptosis. These results suggest the possible involvement of multiple signaling pathways from the MAPK to the subsequent mitochondria- and/or Fas-mediated caspase activation are potential requirements for PCA-induced AGS apoptosis. Further, PCA effectively induced JNK/p38 activation in PCA-response cell lines. Taken together, our data present the first evidence of PCA as an apoptosis inducer in AGS cells, even in tumor cells of digestive organs, and provide a new mechanism for its anticancer activity.
Protection against Adriamycin cytotoxicity and inhibition of DNA topoisomerase II activity by 3,4-dihydroxybenzoic acid.[Pubmed:12792789]
Int J Oncol. 2003 Jul;23(1):159-63.
The mechanism of Adriamycin (ADR) induced cytotoxicity is not completely understood. While a variety of mechanisms have been proposed, the production of free radicals by redox cycling of the semiquinone has been implicated in cytotoxicity, specifically for cardiotoxicity. To determine whether a scavenger of free radicals would modify the cytotoxicity of ADR, the benzoic acid derivative 3,4-Dihydroxybenzoic acid (DHB) was investigated for its ability to protect against ADR-induced cytotoxicity and DNA double strand breaks in Chinese hamster V79 cells. V79 cells were treated with ADR, or its non-redox cycling analog iminodaunomycin, in the presence or absence of DHB. DHB provided significant protection (dose-modifying factor greater than 2.5 for ADR, and nearly 2 for iminodaunomycin) and also caused a dose-dependent decrease in DNA double strand breaks as measured by pulsed field gel electrophoresis. Assays of topoisomerase II activity showed that DHB inhibited topoisomerase II in a concentration-dependent manner, but did not inhibit topoisomerase I. Another non-toxic topoisomerase II inhibitor, the radioprotector WR-1065, also protected against ADR-induced cytotoxicity. These data identify DHB as a non-toxic inhibitor of DNA topoisomerase II and suggest that much of the cytotoxicity of ADR in actively growing V79 cells is due to mechanisms other than redox cycling by the semiquinone.
Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita.[Pubmed:23603737]
Microb Pathog. 2013 Jun-Jul;59-60:52-9.
In this study, the 3,4-Dihydroxybenzoic acid (3,4-DHBA) from Terminalia nigrovenulosa bark (TNB) was purified and its in vitro nematicidal activity was investigated against Meloidogyne incognita. The purification of 3,4-DHBA used a silica gel column and Sephadex LH-20 chromatography combined with thin-layer chromatography and high performance liquid chromatography. Structural identification of the 3,4-DHBA was conducted using (1)H nuclear magnetic resonance (NMR), (13)C NMR, and liquid chromatography time-of-flight mass spectrometry. Nematicidal activity bioassays revealed that 3,4-DHBA treatment resulted in 33.3, 47.5, 72.5 and 94.2% J2 mortality at 0.125, 0.25, 0.5 and 1.0 mg/ml, respectively after 12 h incubation. J2 mortality was increased significantly (P < 0.0001) with increasing incubation time in the range of 54.2-94.2% from 3 to 9 h after incubation with 3,4-DHBA (1.0 mg/ml), but with no significant difference observed where the incubation time was increased from 9 to 12 h. The 3,4-DHBA treatment resulted in 33.3, 65.0, 76.7 and 85.0% hatch inhibition at 0.125, 0.25, 0.5 and 1.0 mg/ml, respectively, 3 days after incubation. Changes in the shape of the eggs were determined after incubation for 1 day with a 3,4-DHBA concentration of 1.0 mg/ml.


