(-)-Epigallocatechin gallateAntioxidant, antiangiogenic and antitumor agent CAS# 989-51-5 |
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- Sotrastaurin (AEB071)
Catalog No.:BCC3857
CAS No.:425637-18-9
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 989-51-5 | SDF | Download SDF |
| PubChem ID | 65064 | Appearance | White powder |
| Formula | C22H18O11 | M.Wt | 458.38 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Epigallocatechin gallate | ||
| Solubility | DMSO : ≥ 30 mg/mL (65.45 mM) H2O : 20 mg/mL (43.63 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
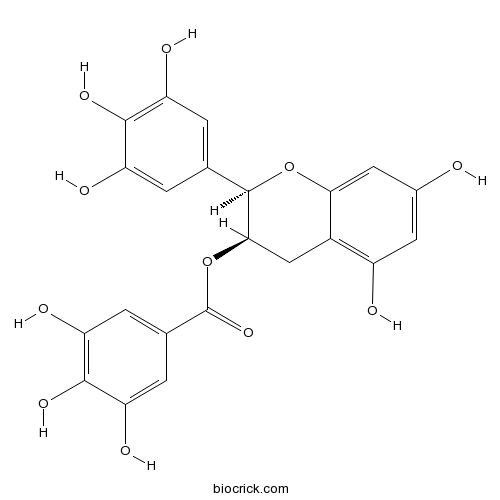
| Chemical Name | [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O | ||
| Standard InChIKey | WMBWREPUVVBILR-WIYYLYMNSA-N | ||
| Standard InChI | InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Epigallocatechin Gallate is an antioxidant polyphenol flavonoid form green tea, and inhibits the activation of EGFR, HER2 and HER3, with antitumor activity. (-)-Epigallocatechin gallate treatment enduring to cardio protection at mitochondrial level, it has protective effects against βA-induced neuronal apoptosis through scavenging reactive oxygen species, which may be beneficial for the prevention of Alzheimer's disease. |
| Targets | Caspase | Bcl-2/Bax | Beta Amyloid | p53 | COX | Bcl-XL |
| In vitro | (-)-Epigallocatechin-gallate (EGCG) stabilize the mitochondrial enzymes and inhibits the apoptosis in cigarette smoke-induced myocardial dysfunction in rats.[Pubmed: 24197690]Mol Biol Rep. 2013 Dec;40(12):6533-45.
|
| In vivo | Modulation of endocrine systems and food intake by green tea epigallocatechin gallate.[Pubmed: 10698173]Endocrinology, 2000, 141(3):980-7.Green tea polyphenols, especially the catechin, (-)-Epigallocatechin gallate (EGCG), have been proposed as a cancer chemopreventative based on a variety of laboratory studies.
|
| Cell Research | The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons.[Pubmed: 11811904]Life Sci. 2001 Dec 21;70(5):603-14.Previous evidence has indicated that the neuronal toxicity of amyloid beta (betaA) protein is mediated through oxygen free radicals and can be attenuated by antioxidants and free radical scavengers. Recent studies have shown that green tea polyphenols reduced free radical-induced lipid peroxidation.
|
| Animal Research | Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice[Reference: WebLink]P. Natl. Acad. Sci. U.S.A., 2002,99(19):12455-60.
|
| Structure Identification | FEBS Lett. 2015 Jan 2;589(1):77-83.The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios.[Pubmed: 25436420]The accumulation of amyloid-beta (Aβ) and tau aggregates is a pathological hallmark of Alzheimer's disease. Both polypeptides form fibrillar deposits, but several lines of evidence indicate that Aβ and tau form toxic oligomeric aggregation intermediates. Depleting such structures could thus be a powerful therapeutic strategy. We generated a fragment of tau (His-K18ΔK280) that forms stable, toxic, oligomeric tau aggregates in vitro. We show that (-)-Epigallocatechin gallate (EGCG), a green tea polyphenol that was previously found to reduce Aβ aggregation, inhibits the aggregation of tau K18ΔK280 into toxic oligomers at ten- to hundred-fold substoichiometric concentrations, thereby rescuing toxicity in neuronal model cells. |

(-)-Epigallocatechin gallate Dilution Calculator

(-)-Epigallocatechin gallate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1816 mL | 10.908 mL | 21.816 mL | 43.6319 mL | 54.5399 mL |
| 5 mM | 0.4363 mL | 2.1816 mL | 4.3632 mL | 8.7264 mL | 10.908 mL |
| 10 mM | 0.2182 mL | 1.0908 mL | 2.1816 mL | 4.3632 mL | 5.454 mL |
| 50 mM | 0.0436 mL | 0.2182 mL | 0.4363 mL | 0.8726 mL | 1.0908 mL |
| 100 mM | 0.0218 mL | 0.1091 mL | 0.2182 mL | 0.4363 mL | 0.5454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
EGCG prevents cardiac mitochondrial metabolism and apoptosis in CS-exposed rats and exhibits enduring cardio protection at mitochondrial level, where it shelters the activities of TCA cycle enzymes and antioxidant enzymes from CS exposure with concomitant decrease in lipid peroxidation and increase in GSH level, inhibits apoptosis through a series of cellular events, including inhibition of the release of cytochrome c into cytosol, activation of pro-caspase-3, down-regulation of Bax and up-regulation of Bcl-2, and reverses the ultra structural apoptotic alternations of mitochondria and nucleus.
Abstract
EGCG, which causes positive results in in vitro mammalian genotoxicity assays via oxidative stress and negative results in in vivo MN studies, was used to assess the effect of cell differentiation status on MN induction and significantly induced the expression of genotoxic response related genes in NHEKs after 12h. The Oxidative stress probably caused by EGCG can be eliminated by EpiDermTM under in vitro experimental conditions.
Abstract
EGCG, a strong antioxidant with possible anti-obesity and anti-diabetic effects, was administered intraperitoneally to alloxan-induced diabetic mice at doses of 50mg/kg body weight for a period of 7 days resulting in a significant increase of body weight and haematological/immunological blood parameters, 100% survival, a significant decrease of lipid peroxidation in liver, kidney and brain tissue, and remarkably reduced DNA damage in peripheral lymphocytes. Due to the demonstrated beneficial effects against diabetes and the associated consequences of free-radical formation in kidney, liver, spleen and brain tissues, EGCG could be used as a dietary supplement potentially contributing to nutritional strategies for the prevention and treatment of diabetes mellitus.
Abstract
EGCG is involved in UVB irradiation-induced autophagy in RPE cells, where the treatment of EGCG significantly reduced the formation of LC3-II and autohagosomes and remarkably relieved UVB irradiation-induced toxicity to RPE cells in an autophagy-dependent manner, and is a potential therapeutic reagent that could be incorporated into the treatment of pathological conditions associated with abnormal autophagy.
Abstract
EGCG, which is hepatotoxic to humans and animals, barely affected oxidative phosphorylation at 7.5-100 μΜ in normal mitochondria of rats liver but remarkably inhibited RCCs in mitochondria undergoing Ca(2+) overload-induced MPT only if IM integrity was compromised. EGCG promotes Ca(2+) overload-induced MPT after moderate MPT has already commenced and worsens pre-existing mitochondria abnormalities triggering hepatotoxicity since it only targets hepatic RCCs in swelling mitochondrial other than normal mitochondrial.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(-)-Epigallocatechin gallate (EGCG), the major catechin accounting for 59% of the total catechins in green tea, is a powerful antioxidant as well as an antiangiogenic and antitumor agent. EGCG has been studied for its role in the chemoprevention of a wild range of cancers, including liver, stomach, skin, lung, mammary gland and colon cancers. Study results show that EGCG is able to induce apoptosis, promote cell growth arrest and block carcinogenesis by affecting signal transduction pathways. Moreover, EGCG exhibits inhibition against a variety of viruses, including HCV, HIV-1, HBV, HSV-1, HSV-2, EBV, adenovirus, influenza virus and enterovirus, as well as several enzymes, including DNMTs, proteases and DHFR.
Reference
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011; 82(12):1807-1821.
Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013; 168(5):1059-1073
- 3-Epiursolic acid
Catalog No.:BCN3173
CAS No.:989-30-0
- Pseudolaric acid A-O-beta-D-glucopyranoside
Catalog No.:BCN1290
CAS No.:98891-44-2
- Pseudolaric acid B-O-beta-D-glucopyranoside
Catalog No.:BCN1291
CAS No.:98891-41-9
- Danshenxinkun D
Catalog No.:BCN2472
CAS No.:98873-76-8
- FLAG tag Peptide
Catalog No.:BCC2562
CAS No.:98849-88-8
- (R)-(+)-Bay K 8644
Catalog No.:BCC7107
CAS No.:98791-67-4
- 3-(4-Hydroxy-3-methoxyphenyl)propyl tetracosanoate
Catalog No.:BCN1292
CAS No.:98770-70-8
- Reboxetine mesylate
Catalog No.:BCC4934
CAS No.:98769-84-7
- Isothymusin
Catalog No.:BCN4532
CAS No.:98755-25-0
- Latifoline N-oxide
Catalog No.:BCN1979
CAS No.:98752-06-8
- Paeonilactone A
Catalog No.:BCN3967
CAS No.:98751-79-2
- Paeonilactone B
Catalog No.:BCN3963
CAS No.:98751-78-1
- Limonol
Catalog No.:BCN4533
CAS No.:989-61-7
- Fmoc-His(Fmoc)-OH
Catalog No.:BCC3500
CAS No.:98929-98-7
- Fmoc-Arg(Mtr)-OH
Catalog No.:BCC3074
CAS No.:98930-01-9
- 3,5-DHBA
Catalog No.:BCC7951
CAS No.:99-10-5
- Ac-DL-Leu-OH
Catalog No.:BCC2977
CAS No.:99-15-0
- Prunasin
Catalog No.:BCN4535
CAS No.:99-18-3
- Trehalose
Catalog No.:BCC9182
CAS No.:99-20-7
- Methyl gallate
Catalog No.:BCN3823
CAS No.:99-24-1
- Chelidonic acid
Catalog No.:BCN6547
CAS No.:99-32-1
- 2-Methyl-5-Isopropenyl-2-Cyclohexenone
Catalog No.:BCC8279
CAS No.:99-49-0
- 3,4-Dihydroxybenzoic acid
Catalog No.:BCN4537
CAS No.:99-50-3
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
Modulation of endocrine systems and food intake by green tea epigallocatechin gallate.[Pubmed:10698173]
Endocrinology. 2000 Mar;141(3):980-7.
Green tea polyphenols, especially the catechin, (-)-Epigallocatechin gallate (EGCG), have been proposed as a cancer chemopreventative based on a variety of laboratory studies. For clear assessment of the possible physiological effects of green tea consumption, we injected pure green tea catechins ip into rats and studied their acute effects on endocrine systems. We found that EGCG, but not related catechins, significantly reduced food intake; body weight; blood levels of testosterone, estradiol, leptin, insulin, insulin-like growth factor I, LH, glucose, cholesterol, and triglyceride; as well as growth of the prostate, uterus, and ovary. Similar effects were observed in lean and obese male Zucker rats, suggesting that the effect of EGCG was independent of an intact leptin receptor. EGCG may interact specifically with a component of a leptin-independent appetite control pathway. Endocrine changes induced by parenteral administration of EGCG may relate to the observed growth inhibition and regression of human prostate and breast tumors in athymic mice treated with EGCG as well as play a role in the mechanism by which EGCG inhibits cancer initiation and promotion in various animal models of cancer.
The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios.[Pubmed:25436420]
FEBS Lett. 2015 Jan 2;589(1):77-83.
The accumulation of amyloid-beta (Abeta) and tau aggregates is a pathological hallmark of Alzheimer's disease. Both polypeptides form fibrillar deposits, but several lines of evidence indicate that Abeta and tau form toxic oligomeric aggregation intermediates. Depleting such structures could thus be a powerful therapeutic strategy. We generated a fragment of tau (His-K18DeltaK280) that forms stable, toxic, oligomeric tau aggregates in vitro. We show that (-)-Epigallocatechin gallate (EGCG), a green tea polyphenol that was previously found to reduce Abeta aggregation, inhibits the aggregation of tau K18DeltaK280 into toxic oligomers at ten- to hundred-fold substoichiometric concentrations, thereby rescuing toxicity in neuronal model cells.
The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons.[Pubmed:11811904]
Life Sci. 2001 Dec 21;70(5):603-14.
Previous evidence has indicated that the neuronal toxicity of amyloid beta (betaA) protein is mediated through oxygen free radicals and can be attenuated by antioxidants and free radical scavengers. Recent studies have shown that green tea polyphenols reduced free radical-induced lipid peroxidation. The purpose of this study was to investigate whether (-)-Epigallocatechin gallate (EGCG) would prevent or reduce the death of cultured hippocampal neuronal cells exposed to betaA because EGCG has a potent antioxidant property as a green tea polyphenol. Following exposure of the hippocampal neuronal cells to betaA for 48 hours, a marked hippocampal neuronal injuries and increases in malondialdehyde (MDA) level and caspase activity were observed. Co-treatment of cells with EGCG to betaA exposure elevated the cell survival and decreased the levels of MDA and caspase activity. Proapoptotic (p53 and Bax), Bcl-XL and cyclooxygenase (COX) proteins have been implicated in betaA-induced neuronal death. However, in this study the protective effects of EGCG seem to be independent of the regulation of p53, Bax, Bcl-XL and COX proteins. Taken together, the results suggest that EGCG has protective effects against betaA-induced neuronal apoptosis through scavenging reactive oxygen species, which may be beneficial for the prevention of Alzheimer's disease.
(-)-Epigallocatechin-gallate (EGCG) stabilize the mitochondrial enzymes and inhibits the apoptosis in cigarette smoke-induced myocardial dysfunction in rats.[Pubmed:24197690]
Mol Biol Rep. 2013 Dec;40(12):6533-45.
The present study brings out the preventive role of (-)-epigallocatechin-gallate (EGCG) on cardiac mitochondrial metabolism and apoptosis in cigarette smoke (CS)-exposed rats. The CS-exposed rats showed significantly decreased activities of TCA cycle enzymes and mitochondrial enzymatic antioxidants, on the other hand, mitochondrial lipid peroxidation was increased and GSH level was decreased. Further, CS exposure was found to induce cardiac apoptosis through release of cytochrome c into the cytosol, cleavage of pro-caspase-3 to active caspase-3, up-regulation of pro-apoptotic (Bax) and down-regulation of antiapoptotic (Bcl-2) molecules. The CS-induced apoptosis was further confirmed by mitochondrial and nuclear ultra structural apoptotic features as evaluated by electron microscopic studies. EGCG supplementation shelters the activities of TCA cycle enzymes and antioxidant enzymes, with concomitant decrease in lipid peroxidation and increase in GSH level. EGCG administration inhibited apoptosis through the inhibition of cytochrome c release into cytosol, activation of pro-caspase-3, down regulation of Bax and significant up regulation of Bcl-2. EGCG reversed the ultra structural apoptotic alterations of mitochondria and nucleus. The present study has provided experimental evidences that the EGCG treatment enduring to cardio protection at mitochondrial level.
Comparison of three amyloid assembly inhibitors: the sugar scyllo-inositol, the polyphenol epigallocatechin gallate, and the molecular tweezer CLR01.[Pubmed:22860214]
ACS Chem Neurosci. 2012 Jun 20;3(6):451-8.
Many compounds have been tested as inhibitors or modulators of amyloid beta-protein (Abeta) assembly in hope that they would lead to effective, disease-modifying therapy for Alzheimer's disease (AD). These compounds typically were either designed to break apart beta-sheets or selected empirically. Two such compounds, the natural inositol derivative scyllo-inositol and the green-tea-derived flavonoid epigallocatechin gallate (EGCG), currently are in clinical trials. Similar to most of the compounds tested thus far, the mechanism of action of scyllo-inositol and EGCG is not understood. Recently, we discovered a novel family of assembly modulators, Lys-specific molecular tweezers, which act by binding specifically to Lys residues and modulate the self-assembly of amyloid proteins, including Abeta, into formation of nontoxic oligomers by a process-specific mechanism (Sinha, S., Lopes, D. H., Du, Z., Pang, E. S., Shanmugam, A., Lomakin, A., Talbiersky, P., Tennstaedt, A., McDaniel, K., Bakshi, R., Kuo, P. Y., Ehrmann, M., Benedek, G. B., Loo, J. A., Klarner, F. G., Schrader, T., Wang, C., and Bitan, G. (2011) Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins. J. Am. Chem. Soc.133, 16958-16969). Here, we compared side-by-side the capability of scyllo-inositol, EGCG, and the molecular tweezer CLR01 to inhibit Abeta aggregation and toxicity. We found that EGCG and CLR01 had comparable activity whereas scyllo-inositol was a weaker inhibitor. Exploration of the binding of EGCG and CLR01 to Abeta using heteronuclear solution-state NMR showed that whereas CLR01 bound to the two Lys and single Arg residues in Abeta monomers, only weak, nonspecific binding was detected for EGCG, leaving the binding mode of the latter unresolved.
Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling.[Pubmed:19826036]
Cancer Res. 2009 Oct 15;69(20):7986-93.
Oncogenes influence nutrient metabolism and nutrient dependence. The oncogene c-Myc stimulates glutamine metabolism and renders cells dependent on glutamine to sustain viability ("glutamine addiction"), suggesting that treatments targeting glutamine metabolism might selectively kill c-Myc-transformed tumor cells. However, many current or proposed cancer therapies interfere with the metabolism of glucose, not glutamine. Here, we studied how c-Myc-transformed cells maintained viability when glucose metabolism was impaired. In SF188 glioblastoma cells, glucose deprivation did not affect net glutamine utilization but elicited a switch in the pathways used to deliver glutamine carbon to the tricarboxylic acid cycle, with a large increase in the activity of glutamate dehydrogenase (GDH). The effect on GDH resulted from the loss of glycolysis because it could be mimicked with the glycolytic inhibitor 2-deoxyglucose and reversed with a pyruvate analogue. Furthermore, inhibition of Akt signaling, which facilitates glycolysis, increased GDH activity whereas overexpression of Akt suppressed it, suggesting that Akt indirectly regulates GDH through its effects on glucose metabolism. Suppression of GDH activity with RNA interference or an inhibitor showed that the enzyme is dispensable in cells able to metabolize glucose but is required for cells to survive impairments of glycolysis brought about by glucose deprivation, 2-deoxyglucose, or Akt inhibition. Thus, inhibition of GDH converted these glutamine-addicted cells to glucose-addicted cells. The findings emphasize the integration of glucose metabolism, glutamine metabolism, and oncogenic signaling in glioblastoma cells and suggest that exploiting compensatory pathways of glutamine metabolism can improve the efficacy of cancer treatments that impair glucose utilization.
EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer.[Pubmed:17981559]
Front Biosci. 2008 Jan 1;13:440-52.
We have shown that epigallocatechin-3-gallate (EGCG), a polyphenolic compound from green tea, inhibits growth and induces apoptosis in human pancreatic cancer cells. However, the preclinical potential of EGCG in a suitable mouse model has not been examined. In this study, we examined the molecular mechanisms by which EGCG inhibited growth, invasion, metastasis and angiogenesis of human pancreatic cancer cells in a xenograft model system. EGCG inhibited viability, capillary tube formation and migration of HUVEC, and these effects were further enhanced in the presence of an ERK inhibitor. In vivo, AsPC-1 xenografted tumors treated with EGCG showed significant reduction in volume, proliferation (Ki-67 and PCNA staining), angiogenesis (vWF, VEGF and CD31) and metastasis (MMP-2, MMP-7, MMP-9 and MMP-12) and induction in apoptosis (TUNEL), caspase-3 activity and growth arrest (p21/WAF1). EGCG also inhibited circulating endothelial growth factor receptor 2 (VEGF-R2) positive endothelial cells derived from xenografted mice. Tumor samples from EGCG treated mice showed significantly reduced ERK activity, and enhanced p38 and JNK activities. Overall, our data suggest that EGCG inhibits pancreatic cancer growth, invasion, metastasis and angiogenesis, and thus could be used for the management of pancreatic cancer prevention and treatment.
Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids.[Pubmed:16037419]
Mol Pharmacol. 2005 Oct;68(4):1018-30.
In the present investigation, we studied the modulating effects of several tea catechins and bioflavonoids on DNA methylation catalyzed by prokaryotic SssI DNA methyltransferase (DNMT) and human DNMT1. We found that each of the tea polyphenols [catechin, epicatechin, and (-)-epigallocatechin-3-O-gallate (EGCG)] and bioflavonoids (quercetin, fisetin, and myricetin) inhibited SssI DNMT- and DNMT1-mediated DNA methylation in a concentration-dependent manner. The IC(50) values for catechin, epicatechin, and various flavonoids ranged from 1.0 to 8.4 microM, but EGCG was a more potent inhibitor, with IC(50) values ranging from 0.21 to 0.47 microM. When epicatechin was used as a model inhibitor, kinetic analyses showed that this catechol-containing dietary polyphenol inhibited enzymatic DNA methylation in vitro largely by increasing the formation of S-adenosyl-L-homocysteine (a potent noncompetitive inhibitor of DNMTs) during the catechol-O-methyltransferase-mediated O-methylation of this dietary catechol. In comparison, the strong inhibitory effect of EGCG on DNMT-mediated DNA methylation was independent of its own methylation and was largely due to its direct inhibition of the DNMTs. This inhibition is strongly enhanced by Mg(2+). Computational modeling studies showed that the gallic acid moiety of EGCG plays a crucial role in its high-affinity, direct inhibitory interaction with the catalytic site of the human DNMT1, and its binding with the enzyme is stabilized by Mg(2+). The modeling data on the precise molecular mode of EGCG's inhibitory interaction with human DNMT1 agrees perfectly with our experimental finding.
Green tea catechins as a BACE1 (beta-secretase) inhibitor.[Pubmed:14592472]
Bioorg Med Chem Lett. 2003 Nov 17;13(22):3905-8.
In the course of searching for BACE1 (beta-secretase) inhibitors from natural products, the ethyl acetate soluble fraction of green tea, which was suspected to be rich in catechin content, showed potent inhibitory activity. (-)-Epigallocatechin gallate, (-)-epicatechin gallate, and (-)-gallocatechin gallate were isolated with IC(50) values of 1.6 x 10(-6), 4.5 x 10(-6), and 1.8 x 10(-6) M, respectively. Seven additional authentic catechins were tested for a fundamental structure-activity relationship. (-)-Catechin gallate, (-)-gallocatechin, and (-)-epigallocatechin significantly inhibited BACE1 activity with IC(50) values of 6.0 x 10(-6), 2.5 x 10(-6), and 2.4 x 10(-6) M, respectively. However, (+)-catechin, (-)-catechin, (+)-epicatechin, and (-)-epicatechin exhibited about ten times less inhibitory activity. The stronger activity seemed to be related to the pyrogallol moiety on C-2 and/or C-3 of catechin skeleton, while the stereochemistry of C-2 and C-3 did not have an effect on the inhibitory activity. The active catechins inhibited BACE1 activity in a non-competitive manner with a substrate in Dixon plots.
Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells.[Pubmed:10775421]
Arch Biochem Biophys. 2000 Apr 15;376(2):338-46.
Green tea has shown remarkable anti-inflammatory and cancer chemopreventive effects in many animal tumor bioassays, cell culture systems, and epidemiological studies. Many of these biological effects of green tea are mediated by epigallocatechin 3-gallate (EGCG), the major polyphenol present therein. We have earlier shown that EGCG treatment results in apoptosis of several cancer cells, but not of normal cells (J. Natl. Cancer Inst. 89, 1881-1886 (1997)). The mechanism of this differential response of EGCG is not known. In this study, we investigated the involvement of NF-kappaB during these differential responses of EGCG. EGCG treatment resulted in a dose-dependent (i) inhibition of cell growth, (ii) G0/G1-phase arrest of the cell cycle, and (iii) induction of apoptosis in human epidermoid carcinoma (A431) cells, but not in normal human epidermal keratinocytes (NHEK). Electromobility shift assay revealed that EGCG (10-80 microM) treatment results in lowering of NF-kappaB levels in both the cytoplasm and nucleus in a dose-dependent manner in both A431 cells and NHEK, albeit at different concentrations. EGCG treatment was found to result in a dose-based differential inhibition of TNF-alpha- and LPS-mediated activation of NF-kappaB in these cells. The inhibition of NF-kappaB constitutive expression and activation in NHEK was observed only at high concentrations. The immunoblot analysis also demonstrated a similar pattern of inhibition of the constitutive expression as well as activation of NF-kappaB/p65 nuclear protein. This inhibition of TNF-alpha-caused NF-kappaB activation was mediated via the phosphorylative degradation of its inhibitory protein IkappaBalpha. Taken together, EGCG was found to impart differential dose-based NF-kappaB inhibitory response in cancer cells vs normal cells; i.e., EGCG-mediated inhibition of NF-kappaB constitutive expression and activation was found to occur at much higher dose of EGCG in NHEK as compared to A431 cells. This study suggests that EGCG-caused cell cycle deregulation and apoptosis of cancer cells may be mediated through NF-kappaB inhibition.


