Cephalotaxus fortunei
Cephalotaxus fortunei
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
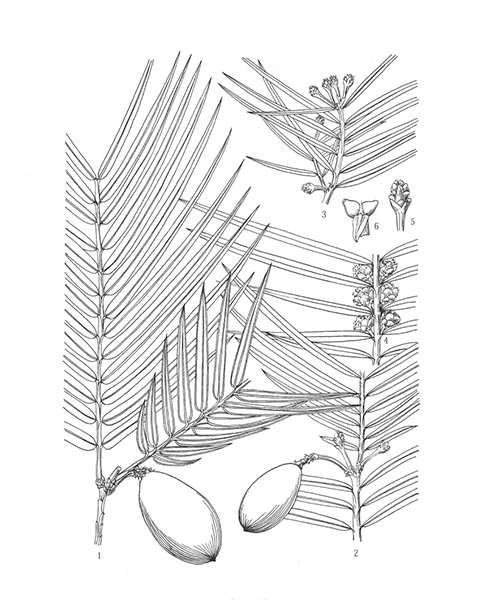
Natural products/compounds from Cephalotaxus fortunei
- Cat.No. Product Name CAS Number COA
-
BCN2957
Cephalotaxine24316-19-6
Instructions

-
BCN6794
Harringtonine26833-85-2
Instructions

-
BCN4958
Homoharringtonine26833-87-4
Instructions

-
BCN6291
Arctigenin7770-78-7
Instructions
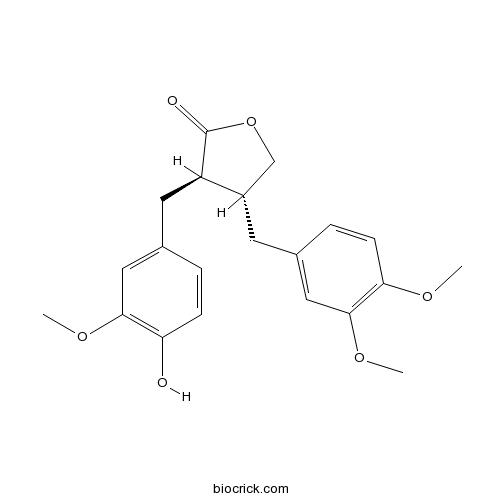
Bioactive norditerpenoids from Cephalotaxus fortunei var. alpina and C. lanceolata.[Pubmed: 29665476]
None
Trichodermadiones A and B from the solid culture of Trichoderma atroviride S361, an endophytic fungus in Cephalotaxus fortunei.[Pubmed: 29626624]
Chemical investigation on the solid rice culture of Trichoderma atroviride S361, an endophyte isolated from Cephalotaxus fortunei, has afforded a pair of novel N-furanone amide enantiomers, (-)-trichodermadione A (1a) and (+)-trichodermadione A (1b), and a new cyclohexenone sesquiterpenoid, trichodermadione B (2), together with six known secondary metabolites. Chiral separation of compound 1 was successfully performed on a Lux Cellulose-2 column. Their structures were elucidated by detailed spectroscopic analyses on the basis of NMR, HRMS, and ECD data, and the absolute configurations of the new compounds were determined by computational analyses of their electronic circular dichroism (ECD) spectra and Snatzke's method. Compounds 1a, 1b and 2 were also evaluated for their cytotoxicity against DU145 and PC3 cell lines, as well as inhibitory effects against the production of NO in LPS-stimulated RAW264.7 cells.
Diterpenoids and Lignans from Cephalotaxus fortunei.[Pubmed: 28139925]
None
Development and characterization of 15 microsatellite markers for Cephalotaxus fortunei (Cephalotaxaceae).[Pubmed: 27213121]
To survey population variation and the adaptive evolution of Cephalotaxus fortunei (Cephalotaxaceae), an endemic and endangered conifer in China, microsatellite markers were developed and characterized for this species.
A new furan derivative from an endophytic Aspergillus flavus of Cephalotaxus fortunei.[Pubmed: 25942282]
A new furan derivative named 5-acetoxymethylfuran-3-carboxylic acid (2), together with a known furan compound, 5-hydroxymethylfuran-3-carboxylic acid (1), were isolated from the fermentation of Aspergillus flavus, endophytic fungi in Cephalotaxus fortunei. The structures of 1 and 2 were elucidated by NMR, IR, UV and MS data, as well as compared with literature data. The compounds 1 and 2 exhibited potent antibacterial activity against Staphylococcus aureus with MIC values of 31.3 and 15.6 μg/mL, respectively. The compound 2 showed moderate antioxidant activity.
Nematotoxicity of drupacine and a Cephalotaxus alkaloid preparation against the plant-parasitic nematodes Meloidogyne incognita and Bursaphelenchus xylophilus.[Pubmed: 23785026]
Species of Cephalotaxus (the plum yews) produce nematotoxic compounds of unknown identity. Consequently, bioassay-guided fractionation was employed to identify the compound(s) in Cephalotaxus fortunei twigs and leaves with activity against plant-parasitic nematodes.
[Chemical properties and enzyme activities of rhizosphere and non-rhizosphere soils under six Chinese herbal medicines on Mt. Taibai of Qinling Mountains, Northwest China].[Pubmed: 23359927]
This paper studied the chemical properties and enzyme activities of rhizosphere and non-rhizosphere soils in different habitats of six Chinese herbal medicines, including Pyrola decorata, Cephalotaxus fortunei, Polygonatum odoratum, Potentilla glabra, Polygonum viviparum, and Potentilla fruticosa, on the Mt. Taibai of Qinling Mountains. In the rhizosphere soils of the herbs, the contents of soil organic matter, total nitrogen, available nitrogen, and available phosphorus and the soil cation exchange capacity (CEC) were higher, presenting an obvious rhizosphere aggregation, and the soil enzyme activities also showed an overall stronger characteristics, compared with those in non-rhizosphere soils. The soil organic matter, total nitrogen, and total phosphorus contents in the rhizosphere soils had significant positive correlations with soil neutral phosphatase activity, and the soil CEC had significant positive correlations with the activities of soil neutral phosphatase and acid phosphatase. In the non-rhizosphere soils, the soil organic matter and total nitrogen contents had significant positive correlations with the activities of soil urease, catalase and neutral phosphatase, and the soil CEC showed a significant positive correlation with the activities of soil urease, catalase, neutral phosphatase and acid phosphatase. The comprehensive fertility level of the rhizosphere soils was higher than that of the non-rhizosphere soils, and the rhizosphere and non-rhizosphere soils of P. fruticosa, P. viviparum, and P. glabra had higher comprehensive fertility level than those of P. decorata, P. odoratum and C. fortunei. In the evaluation of the fertility levels of rhizosphere and non-rhizosphere soils under the six Chinese herbal medicines, soil organic matter content and CEC played important roles, and soil neutral phosphatase could be the preferred soil enzyme indicator.


