Cratoxylum cochinchinense
Cratoxylum cochinchinense
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Cratoxylum cochinchinense
- Cat.No. Product Name CAS Number COA
-
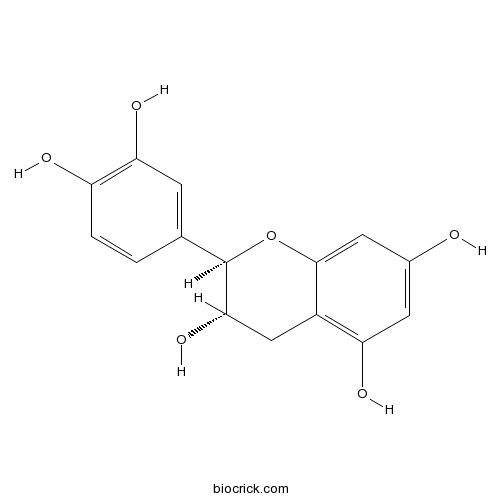
BCN1688
Catechin154-23-4
Instructions
-
BCN1213
Beta-mangostin20931-37-7
Instructions

-
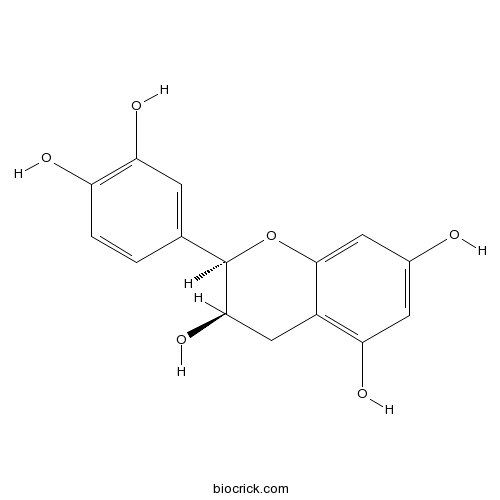
BCN5597
Epicatechin490-46-0
Instructions
Caged xanthones displaying protein tyrosine phosphatase 1B (PTP1B) inhibition from Cratoxylum cochinchinense.[Pubmed: 29533213]
None
Inhibition of protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase by xanthones from Cratoxylum cochinchinense, and their kinetic characterization.[Pubmed: 29306546]
None
Four New Xanthones from Cratoxylum cochinchinense and Their In Vitro Antiproliferative Effects.[Pubmed: 28158891]
None
Anti-Proliferation and Apoptosis Induction in Breast Cancer Cells by Cratoxylum cochinchinense Extract.[Pubmed: 29901920]
Breast cancer is the most common invasive cancer in females worldwide. It was found about 37.5% in Thai females and is one of the leading causes of death-related cancers in women. Therefore, new finding of anti-cancer compound as a therapeutic candidate in breast cancer is necessary.
Bioactive Xanthones from Cratoxylum cochinchinense.[Pubmed: 26749839]
Chemical investigation of Cratoxylum cochinchinense stem bark has led to the isolation and identification of a new xanthone, cochinchinone M (1), together with 12 known compounds. Their structures were elucidated on the basis of spectroscopic methods, including UV, IR, NMR and MS. Some compounds were evaluated for their antibacterial and acetylcholinesterase inhibitory activities.
Bioactive prenylated xanthones from the stems of Cratoxylum cochinchinense.[Pubmed: 26043754]
Cochinchinones M-U (1-9), together with 12 known compounds (10-21), were isolated from the stems of Cratoxylum cochinchinense (Lour.) Blume. Their structures were determined on the basis of extensive spectroscopic data analyses. In addition, their retinoid X receptor-α transcriptional activities were evaluated using an in vitro assay.
A new bisanthraquinone and cytotoxic xanthones from Cratoxylum cochinchinense.[Pubmed: 24571674]
A new bisanthraquinone has been isolated from the stems of Cratoxylum cochinchinense together with vismiaquinone C and 16 known xanthones. Their structures were characterised by using spectroscopic methods. Two of the isolated compounds are modified xanthones (6 and 15) and exhibited strong cytotoxicity against human epidermoid carcinoma (A341: IC50 2.01, 1.78 μg/mL) and human breast cancer cell lines (SKBR3: IC50 1.54, 0.69 μg/mL).
Xanthones from the stems of Cratoxylum cochinchinense.[Pubmed: 22865675]
Four new xanthones, 1-methoxy-3,7,8-trihydroxyxanthone (1), 1-methoxy-4,7,8-trihydroxyxanthone (2), 1-methoxy-4,7-dihydroxyxanthone (3), and 1,4-dimethoxy-2,7-dihydroxyxanthone (4) were isolated from the stems of Cratoxylum cochinchinense along with four known xanthones (5-8). The structures of new compounds were determined by extensive spectroscopic analyses, mainly 1D and 2D NMR and HRESIMS data.
Cytotoxic and NF-κB inhibitory constituents of the stems of Cratoxylum cochinchinense and their semisynthetic analogues.[Pubmed: 21428375]
A new caged xanthone (1), a new prenylxanthone (2), seven known xanthones, and a known sterol glucoside were isolated from the stems of Cratoxylum cochinchinense, collected in Vietnam. Compounds 1 and 2 were determined structurally by analysis of their spectroscopic data. In addition, five new (10 and 16-19) and eight known prenylated xanthone derivatives were synthesized from the known compounds α-mangostin (3) and cochinchinone A (6). Several of these substances were found to be cytotoxic toward HT-29 human colon cancer cells, with the most potent being 3,6-di-O-acetyl-α-mangostin (8, ED50, 1.0 μM), which was tested further in an in vivo hollow fiber assay, but found to be inactive at the highest dose used (20 mg/kg; ip). Of the substances evaluated in a NF-κB p65 inhibition assay, 1,3,7-trihydroxy-2,4-diisoprenylxanthone (5) exhibited the most potent activity (IC50, 2.9 μM). In a mitochondrial transmembrane potential assay, two new compounds, 1 (IC50, 3.3 μM) and 10 (IC50, 1.4 μM), and two known compounds, 3 (α-mangostin, IC50, 0.2 μM) and 11 (3,6-di-O-methyl-α-mangostin, IC50, 0.9 μM), were active. A preliminary analogue development study showed that 3,6-diacetylation and 6-benzoylation both slightly increased the cytotoxicity of α-mangostin (3), whereas methylation reduced such activity. In contrast, neither acetylation, benzoylation, nor methylation enhanced the cytotoxicity of cochinchinone A (6).
Xanthones from the stems of Cratoxylum cochinchinense.[Pubmed: 20570298]
Three xanthones, named cratoxylumxanthones B-D (1-3), along with five known xanthones (4-8), were isolated from the stems of Cratoxylum cochinchinense (Lour.) Blume. Their structures were elucidated by interpretation of spectroscopic data. Among these xanthones, cochinxanthone D (4) exhibited the most potent antioxidant activity in both the DPPH radical scavenging and the lipid peroxidation inhibition assays.


