Dactylicapnos scandens
Dactylicapnos scandens
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Dactylicapnos scandens
- Cat.No. Product Name CAS Number COA
-
BCN6165
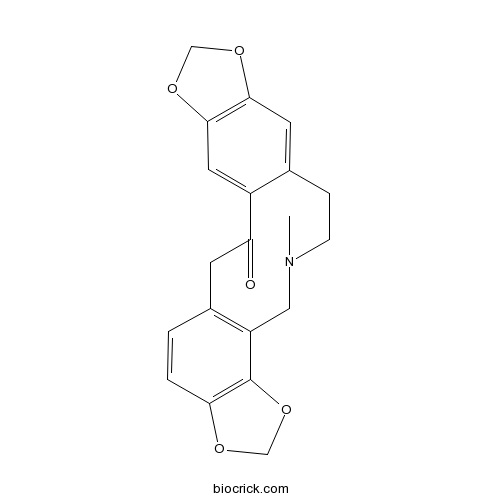
Protopine130-86-9
Instructions
Anti-Inflammatory Isoquinoline with Bis- seco-aporphine Skeleton from Dactylicapnos scandens.[Pubmed: 29508621]
Dactyllactone A (1), which was isolated from Dactylicapnos scandens, is an isoquinoline alkaloid with a rearranged and reconstructed D ring, making it the first of a new subtype of aporphines. Compound 1 might be derived from a common aporphine skeleton, which may have undergone biogenetic rearrangement and cleavage of the aromatic ring. Its structure was determined by extensive spectroscopic analysis and single-crystal X-ray diffraction. Compound 1 exhibited anti-inflammatory activity in vitro significantly by inhibiting the expression of IL-1β and PGE2 in a dose-dependent manner.
Identification of candidate genes involved in isoquinoline alkaloids biosynthesis in Dactylicapnos scandens by transcriptome analysis.[Pubmed: 28831066]
Dactylicapnos scandens (D. Don) Hutch (Papaveraceae) is a well-known traditional Chinese herb used for treatment of hypertension, inflammation, bleeding and pain for centuries. Although the major bioactive components in this herb are considered as isoquinoline alkaloids (IQAs), little is known about molecular basis of their biosynthesis. Here, we carried out transcriptomic analysis of roots, leaves and stems of D. scandens, and obtained a total of 96,741 unigenes. Based on gene expression and phylogenetic relationship, we proposed the biosynthetic pathways of isocorydine, corydine, glaucine and sinomenine, and identified 67 unigenes encoding enzymes potentially involved in biosynthesis of IQAs in D. scandens. High performance liquid chromatography analysis demonstrated that while isocorydine is the most abundant IQA in D. scandens, the last O-methylation biosynthesis step remains unclear. Further enzyme activity assay, for the first time, characterized a gene encoding O- methyltransferase (DsOMT), which catalyzes O-methylation at C7 of (S)-corytuberine to form isocorydine. We also identified candidate transcription factor genes belonging to WRKY and bHLH families that may be involved in the regulation of IQAs biosynthesis. Taken together, we first provided valuable genetic information for D. scandens, shedding light on candidate genes involved in IQA biosynthesis, which will be critical for further gene functional characterization.
Application of a liquid chromatography-tandem mass spectrometry method to the pharmacokinetics, tissue distribution and excretion studies of Dactylicapnos scandens in rats.[Pubmed: 23245239]
The herbal ingredients of isocorydine and protopine were isolated from Dactylicapnos scandens. This study was aimed at developing a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to quantify isocorydine and protopine in rat plasma and tissues for pharmacokinetic, tissue distribution and excretion studies. Biological samples were processed with ethyl acetate extraction, and corydaline was chosen as the internal standard (IS). The analytes were separated by a C(18) column and detected with a triple quadrupole mass spectrometer using positive ion ESI in the multiple reaction monitoring (MRM) mode. The MS/MS ion transitions monitored were m/z 342.0→278.9 for isocorydine, 354.1→188.0 for protopine and 370.0→192.0 for IS, respectively. Excellent linearity was observed over the concentration range between 10 and 3000 ng/mL for isocorydine and 10-300 ng/mL for protopine. The lower limit of quantification (LLOQ) was 10 ng/mL for both isocorydine and protopine. This novel method was rapid, accurate, high sensitive and high selective. It was successfully applied to the pharmacokinetic, tissue distribution and excretion studies of D. scandens. These preclinical data of D. scandens would be useful for the clinical reference.
Preparative isolation of alkaloids from Dactylicapnos scandens using pH-zone-refining counter-current chromatography by changing the length of the separation column.[Pubmed: 22056347]
pH-Zone-refining counter-current chromatography was successfully applied for the preparative separation of alkaloids from Dactylicapnos scandens. The two-phase solvent system was composed of petroleum ether-ethyl acetate-methanol-water (3:7:1:9, v/v), where 20 mM of triethylamine (TEA) was added to the upper phase as a retainer and 5 mM of hydrochloric acid (HCl) to the aqueous phase as an eluter. In this experiment, the apparatus with an adjustable length of the separation column was used for the separation of alkaloids from D. scandens and the resolution of the compounds can be remarkably improved by increasing the length of the separation column. As a result, 70 mg protopin, 30 mg (+) corydine, 120 mg (+) isocorydine and 40 mg (+) glaucine were obtained from 1.0 g of the crude extracts and each with 99.2%, 96.5%, 99.3%, 99.5% purity as determined by HPLC. The chemical structures of these compounds were confirmed by positive ESI-MS and (1)H NMR.
[Alkaloids from Dactylicapnos scandens Hutch].[Pubmed: 19938545]
To investigate the alkaloids in the roots of Dactylicapnos scanden (D. Don) Hutch.
Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models.[Pubmed: 16530230]
The protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. 5-hydroxy-DL-tryptophan(5-HTP)-induced head twitch response (HTR) and tail suspension test were adopted to study whether protopine has anti-depression effect in mice using reference antidepressant fluoxetine and desipramine as positive controls. In HTR test, protopine at doses of 5, 10, 20 mg/kg dose dependently increase the number of 5-HTP-induced HTR. Protopine at doses of 3.75 mg/kg, 7.5 mg/kg and 30 mg/kg also produces a dose-dependent reduction in immobility in the tail suspension test. The present results open up new possibilities for the use of protopine in the treatment of mood disorders, such as mild and moderate states of depression.
[Determination of protopine and isocorydine in root of Dactylicapnos scandens by HPLC].[Pubmed: 15631083]
To establish a HPLC method for determination of protopine and isocorydine in root of Dactylicapnos scandens.
[Pharmacognostical studies on Dactylicapnos scandens].[Pubmed: 15139122]
To offer evidences for quality control of medicinal plant of Dactylicapnos scandens.


