Daphne genkwa
Daphne genkwa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
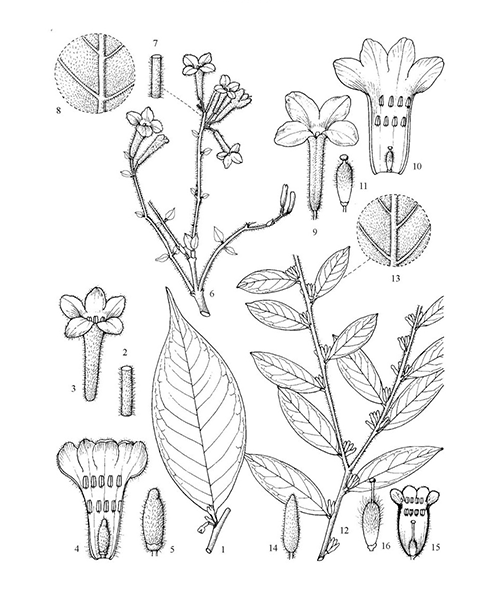
Natural products/compounds from Daphne genkwa
- Cat.No. Product Name CAS Number COA
-
BCN4885
Hydroxygenkwanin20243-59-8
Instructions

-
BCN7834
Clemaphenol A362606-60-8
Instructions
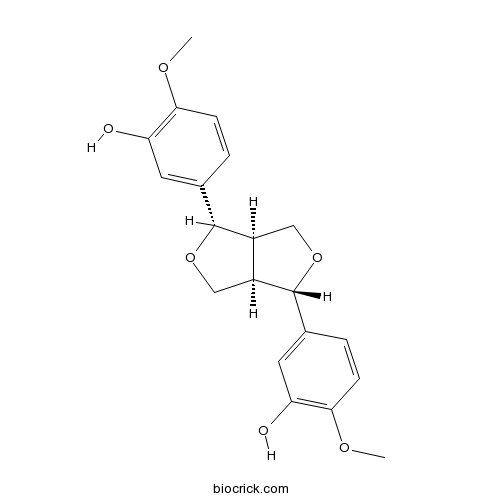
-
BCN1083
Eleutheroside E39432-56-9
Instructions
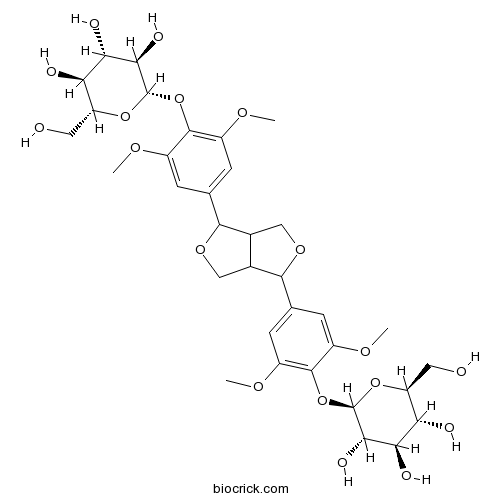
-
BCN5488
Genkwanin437-64-9
Instructions

-
BCN9061
(±)-Naringenin67604-48-2
Instructions
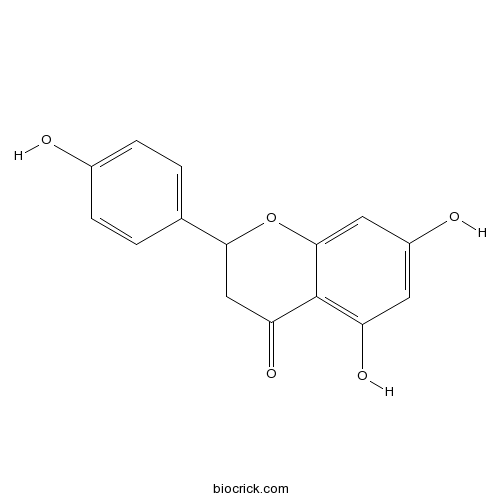
[Vinegar processing attenuates toxicity on IEC-6 cells caused by chloroform extraction of Daphne genkwa].[Pubmed: 29945380]
None
Simultaneous determination of three flavonoids and one coumarin by LC-MS/MS: Application to a comparative pharmacokinetic study in normal and arthritic rats after oral administration of Daphne genkwa extract.[Pubmed: 29500935]
None
[Morphological and microscopical identification of Genkwa Ramulus and its adulterants].[Pubmed: 29493144]
The purpose of this article is to identify Daphne genkwa and its adulterants, Wikstroemia chamaedaphne, according to the morphological and microstructure characteristics of their stem and foliage. The root of D.genkwa was studied simultaneously. The results indicated that the crude drug and processed pieces of Genkwa Ramulus were mainly composed of stems and branches where obvious opposite petiole scars and branch marks were able to be seen on their nodes. Otherwise, foliage or peduncles generally couldn't be found. Moreover, the fine silver flocculent fibers could be observed in the bark of fracture surface. The adulterants were the plant segments which were composed of stems, foliage and peduncles with spikelet-pedicel scars. There existed microstructures differences between Genkwa Ramulus and its adulterants. In the former, single thick lignified phloem fibers were interspersed in the stem phloem of the transverse section with very thick wall and unicellular non-glandular hairs could be observed on the lower epidermis of foliage. Nevertheless, in the latter, there was no thick lignified phloem fibers in cross section of stem phloem, the outer wall of epidermal cells of foliage hadthick cuticles and no non-glandular hairs in lower epidermis of foliage. The results can be used for the identification and the quality standard of the crude drug and processed pieces of D.genkwa.The characteristics of the microstructures and the transverse section can be used to identify the radix D.genkwa.
Pharmacokinetic comparisons of six components from raw and vinegar-processed Daphne genkwa aqueous extracts following oral administration in rats by employing UHPLC-MS/MS approaches.[Pubmed: 29428673]
None
Daphnane and Phorbol Diterpenes, Anti-neuroinflammatory Compounds with Nurr1 Activation from the Roots and Stems of Daphne genkwa.[Pubmed: 29199243]
None
Genkwalathins A and B, new lathyrane-type diterpenes from Daphne genkwa.[Pubmed: 29156984]
None
Absorption Properties of Luteolin and Apigenin in Genkwa Flos Using In Situ Single-Pass Intestinal Perfusion System in the Rat.[Pubmed: 29121796]
The flower bud of Daphne genkwa (Genkwa Flos) is a commonly used herbal medicine in Asian countries. Luteolin and apigenin are two recognized active flavonoids in Genkwa Flos. The aim of this study was to investigate the intestinal absorption mechanisms of Genkwa Flos flavonoids using in situ single-pass intestinal perfusion rat model. Using HPLC, we determined its major effective flavonoids luteolin, apigenin, as well as, hydroxygenkwanin and genkwanin in biological samples. The intestinal absorption mechanisms of the total flavonoids in Genkwa Flos (TFG) were investigated using in situ single-pass intestinal perfusion rat model. Comparing the TFG absorption rate in different intestinal segments, data showed that the small intestine absorption was significantly higher than that of the colon ([Formula: see text]). Compared with duodenum and ileum, the jejunum was the best small intestinal site for TFG absorption. The high TFG concentration (61.48[Formula: see text][Formula: see text]g/ml) yielded the highest permeability ([Formula: see text]). Subsequently, three membrane protein inhibitors (verapamil, pantoprazole and probenecid) were used to explore the TFG absorption pathways. Data showed probenecid, a multidrug resistance protein (or MRP) inhibitor, effectively enhanced the TFG absorption ([Formula: see text]). Furthermore, by comparing commonly used natural absorption enhancers on TFG, it was observed that camphor was the most effective. In Situ single-pass intestinal perfusion experiment shows that TFG absorption is much higher in the small intestine than in the colon, and the TFG is absorbed mainly via an active transport pathway with MRP-mediated efflux mechanism. Camphor obviously enhanced the TFG absorption, and this could be an effective TFG formulation preparation method to increase clinical effectiveness after Genkwa Flos administration. Our study elucidated the TFG absorption mechanisms, and provided new information for its formulation preparation.


