Desmos chinensis
Desmos chinensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Desmos chinensis
- Cat.No. Product Name CAS Number COA
-
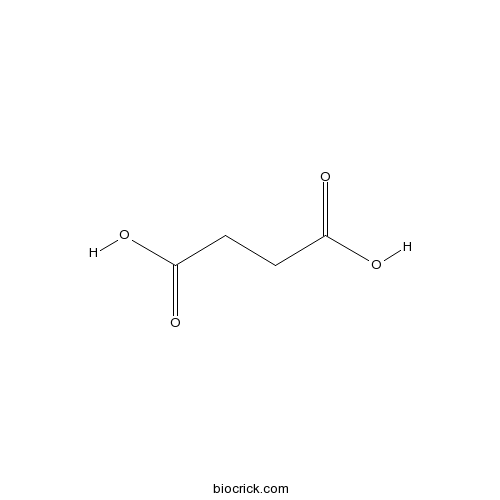
BCN5890
Succinic acid110-15-6
Instructions
The composition and antimicrobial activities of Cyperus conglomeratus, Desmos chinensis var. lawii and Cyathocalyx zeylanicus essential oils.[Pubmed: 22799103]
The essential oil compositions of the rhizomes of Cyperus conglomeratus (Cyperaceae) collected from Oman and the leaves of two Annonaceae plants, Desmos chinensis var. lawii and Cyathocalyx zeylanicus collected from India were studied by GC, GC-MS and 13C NMR spectroscopy. Twenty-six compounds, representing 84.4% of the oil were identified in C. conglomeratus, where eugenol (31.3%), alpha-cyperone (10.5%) and cyperotundone (8.4%) were the major compounds. Twelve compounds, constituting 100%, were identified in D. lawii oil, of which benzyl benzoate (58.7%), beta-caryophyllene (23.2%), limonene (4.9%) and alpha-humulene (4.0%) were the major constituents. Thirty-two compounds, comprising 98.0%, were identified in C. zeylanicus oil, of which beta-caryophyllene (21.6%), alpha-pinene (20.4%) and E-beta-ocimene (11.8%) were the major components. The antibacterial and antifungal activities of the oils were tested against a panel of five bacterial and two fungal strains. The oils showed moderate activity against all the tested microbial strains. The minimum inhibitory concentrations of the oils were also determined.
Phenolic constituents with inhibitory activity against NFAT transcription from Desmos chinensis.[Pubmed: 16392667]
Six phenolic constituents, 2-methoxybenzyl benzoate (1), negletein (2), 2',3'-dihydroxy-4',6'-dimethoxydihydrochalcone (3), 5,6-dihydroxy-7-methoxy-dihydroflavone (4), astilbin (5), and quercitrin (6) were isolated from the methanol extract of the dried leaves of Desmos chinensis. Their structures were elucidated from spectral and chemical data. Of these constituents, compounds 2 (IC50: 3.89 +/- 0.39 microM) and 3 (IC50: 9.77 +/- 0.26 microM) exhibited potent inhibitory activity against nuclear factor of activated T cells (NFAT) transcription factor, and compound 1 (IC50: 28.4 +/- 2.62 microM) exhibited moderate inhibitory activity.
A new C-benzylated chalcone from Desmos chinensis.[Pubmed: 12837375]
A new C-benzylated chalcone was isolated from the leaves of Desmos chinensis. Its structure was established as 2',4'-dihydroxy-3'-(2,6-dihydroxybenzyl)-6'-methoxychalcone on the basis of spectroscopic data.
[Studies on chemical constituents of seeds of Desmos chinensis Lour].[Pubmed: 12205876]
To investigate the chemical constituents of Desmos chinensis.
Isolation of a novel substrate-competitive tyrosine kinase inhibitor, desmal, from the plant Desmos chinensis.[Pubmed: 8458434]
In the course of a screening program for tyrosine kinase inhibitors, the chloroform extract of a tropical plant, Desmos chinensis, strongly inhibited the enzyme activity. The active substance was purified by silica gel, gel filtration, and finally crystallized. The structure was elucidated by mass spectrometry and X-ray crystallography to be 8-formyl-2,5,7-trihydroxy-6- methylflavanone, and we named it desmal. Desmal competed with peptide substrate and non-competed with ATP. It inhibited tyrosine kinase in situ in epidermal growth factor (EGF) receptor-overexpressing NIH3T3 (ER12) cells. It also inhibited EGF-induced inositol phosphate formation and morphological changes.


