Dicranostigma leptopodum
Dicranostigma leptopodum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Dicranostigma leptopodum
- Cat.No. Product Name CAS Number COA
-
BCN6165
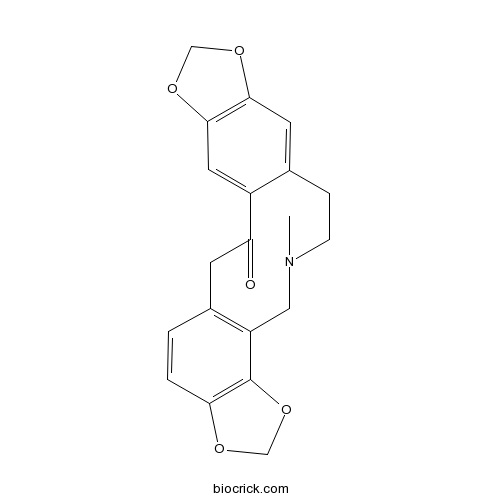
Protopine130-86-9
Instructions
[Research on anticancer activity of isocorydine and its derivatives].[Pubmed: 29171235]
Isocorydine and its analogs were extracted from Dicranostigma leptopodum and Stephania yunnanensis through the method of natural products chemistry. Its derivatives were prepared by chemical structure modifications from isocorydine. MTT method was used to study the inhibitory effect of those compounds on the growth of HepG2, HeLa and MGC-803 cancer cell lines in vitro. The results showed that isocorydine and its analogs all have the growth inhibition for those cancer cell lines. This paper investigated the structure-activity relationship of isocorydine and its derivatives with anticancer activity in the aspect of stereochemical structure, functional groups positions of the compounds and the electron density of aromatic rings based on the single crystal diffraction structure and the molecular docking of EGFR and isocorydine.
Three-phase solvent systems for the comprehensive separation of a wide variety of compounds from Dicranostigma leptopodum by high-speed counter-current chromatography.[Pubmed: 25864484]
A three-phase solvent system was efficiently applied for high-speed counter-current chromatography to separate secondary metabolites with a wide range of hydrophobicity in Dicranostigma leptopodum. The three-phase solvent system of n-hexane/methyl tert-butyl ether/acetonitrile/0.5% triethylamine (2:2:3:2, v/v/v/v) was selected for high-speed counter-current chromatography separation. The separation was initiated by filling the column with a mixture of intermediate phase and lower phase as a stationary phase followed by elution with upper phase to separate the hydrophobic compounds. Then the mobile phase was switched to the intermediate phase to elute the moderately hydrophobic compounds, and finally the polar compounds still retained in the column were fractionated by eluting the column with the lower phase. In this research, 12 peaks were eluted out in one-step operation within 110 min, among them, eight compounds with acceptable purity were obtained and identified. The purities of β-sitosterol, protopine, allocryptopine, isocorydione, isocorydine, coptisine, berberrubine, and berberine were 94.7, 96.5, 97.9, 86.6, 98.9, 97.6, 95.7, and 92.8%, respectively.
Simultaneous determination of the content of isoquinoline alkaloids in Dicranostigma leptopodum (Maxim) Fedde and the effective fractionation of the alkaloids by high-performance liquid chromatography with diode array detection.[Pubmed: 25330407]
A simple and efficient method was developed for the simultaneous determination of eight isoquinoline alkaloids in methanol extracts of Dicranostigma leptopodum (Maxim) Fedde and the effective fractionation of the alkaloids of D. leptopodum by high-performance liquid chromatography with diode array detection. The chromatographic conditions were optimized on a SinoChrom ODS-BP column to obtain a good separation of the four types of alkaloid analytes, including two aporphines (isocorydine, corydine), two protopines (protopine and allocryptopine), a morphine (sinoacutine), and three quaternary protoberberine alkaloids (berberrubine, 5-hydroxycoptisine, and berberine). The separation of these alkaloids was significantly affected by the composition of the mobile phase, and particularly by its pH value. Acetonitrile (A) and 0.2% phosphoric acid solution adjusted to pH 6.32 with triethylamine (B) were selected as the mobile phase with a gradient elution. With this method, a new quaternary protoberberine alkaloid was isolated and the two structural isomers (isocorydine and corydine) were baseline separated. The appropriate harvest period for D. leptopodum was also recommended based on our analysis. The method for the effective fraction of the alkaloids of D. leptopodum was optimized under this method with regard to the varying significant pharmacological activities of the alkaloids.
Cytotoxicity of Aporphine, Protoberberine, and Protopine Alkaloids from Dicranostigma leptopodum (Maxim.) Fedde.[Pubmed: 24963327]
Nine alkaloids with three different structural skeletons were isolated from Dicranostigma leptopodum (Maxim.) Fedde (Papaveraceae) by repeated silica gel column chromatography. Their chemical structures were identified on the basic of physicochemical and spectroscopic data. Among them, 10-O-methylhernovine (1), nantenine (2), corytuberine (3), lagesianine A (4), and dihydrocryptopine (9) were first isolated from this plant. With a series of cytotoxic tests, compounds 2, 3, and 7 displayed cytotoxicity against SMMC-7721 with IC50 values of 70.08 ± 4.63, 73.22 ± 2.35, and 27.77 ± 2.29 μ M, respectively.
A new quaternary protoberberine alkaloid isolated from Dicranostigma leptopodum (Maxim) Fedde.[Pubmed: 24499388]
Phytochemical investigation of the whole plants of Dicranostigma leptopodum (Maxim) Fedde has led to the isolation of two quaternary protoberberine alkaloids 5-hydroxy-coptisine (1) and berberrubine (2). This type of alkaloids was isolated from the genus Dicranostigma for the first time and the new compound structure (1) was elucidated by various spectroscopic methods including 2D NMR techniques (gCOSY, HMQC and HMBC) and HR-ESI-MS.
[Extraction and purification of isocorydine from Dicranostigma leptopodum].[Pubmed: 24218977]
To optimize the conditions of extraction and purification of isocorydine from Dicranostigma leptopodum.
New hopane triterpene from Dicranostigma leptopodum (Maxim) Fedde.[Pubmed: 20390749]
Phytochemical investigation of the whole plant of Dicranostigma leptopodum (Maxim) Fedde led to the isolation of a new hopane triterpene, dicranostigmone (1), and a known compound, erythrodiol-3-O-palmitate (2). The structure of the new compound (1) was elucidated by various spectroscopic methods including 2D NMR techniques (gCOSY, HMQC, HMBC, NOESY), HR-ESI-MS, and X-ray.


