Dioscorea zingiberensis
Dioscorea zingiberensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Dioscorea zingiberensis
- Cat.No. Product Name CAS Number COA
-
BCC8933
Deltonin55659-75-1
Instructions
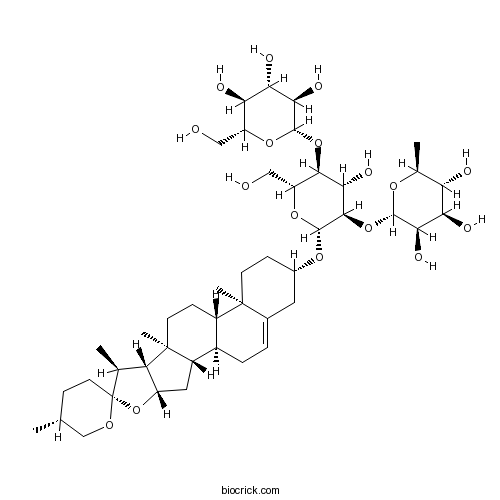
-
BCN2942
Zingiberen newsaponin91653-50-8
Instructions
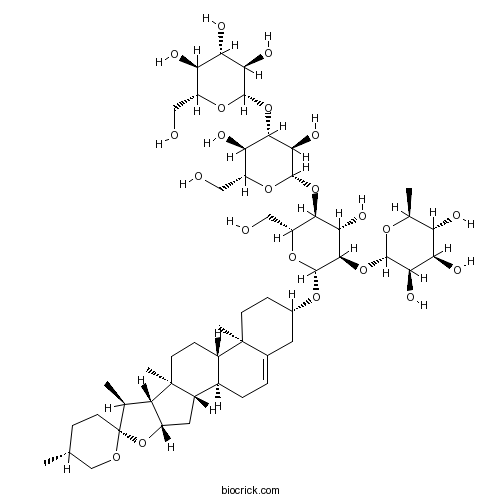
Genome survey sequencing of Dioscorea zingiberensis.[Pubmed: 29883551]
Dioscorea zingiberensis (Dioscoreceae) is the main plant source of diosgenin (steroidal sapogenins), the precursor for the production of steroid hormones in the pharmaceutical industry. Despite its large economic value, genomic information of the genus Dioscorea is currently unavailable. Here, we present an initial survey of the D. zingiberensis genome performed by next-generation sequencing technology together with a genome size investigation inferred by flow cytometry. The whole genome survey of D. zingiberensis generated 31.48 Gb of sequence data with approximately 78.70× coverage. The estimated genome size is 800 Mb, with a high level of heterozygosity based on K-mer analysis. These reads were assembled into 334 288 contigs with a N50 length of 1079 bp, which were further assembled into 92 163 scaffolds with a total length of 173.46 Mb. A total of 4935 genes, 81 tRNAs, 69 rRNAs, and 661 miRNAs were predicted by the genome analysis, and 263 484 repeated sequences were obtained with 419 372 simple sequence repeats (SSRs). Among these SSRs, the mononucleotide repeat type was the most abundant (up to 54.60% of the total SSRs), followed by the dinucleotide (29.60%), trinucleotide (11.37%), tetranucleotide (3.53%), pentanucleotide (0.65%), and hexanucleotide (0.25%) repeat types. The 1C-value of D. zingiberensis was calibrated against Salvia miltiorrhiza and calculated as 0.87 pg (851 Mb) by flow cytometry, which was very close to the result of the genome survey. This is the first report of genome-wide characterization within this taxon.
Eco-friendly microbial production of diosgenin from saponins in Dioscorea zingiberensis tubers in the presence of Aspergillus awamori.[Pubmed: 29750996]
A novel microbial procedure was proposed for diosgenin production from Dioscorea zingiberensis C.H. Wright (DZW) tubers via employing Aspergillus awamori for the first time. The optimal conditions of fermenter cultivation were established as inoculation dosage of 8%, fermentation temperature of 30 °C, cultivation time of 8 days, initial pH of 7.0 and a stirring rate of 180 rpm when the converted diosgenin content reached a peak value of 74.26 ± 3.23 mg/g substrate. The product was purified by silica gel column and then confirmed as diosgenin (purity: 96.9 ± 2.42%) by nuclear magnetic resonance (NMR). Compared with traditional acid hydrolysis, this new process generated indeed less wastewater with lower chemical oxygen demand (COD) reduced to 500 mg/L from 10,000 mg/L and absence of acid and alkali. This research provided definitely an environmental and high-efficiency microbial technology for diosgenin production.
Isolation of endophytic fungi from Dioscorea zingiberensis C. H. Wright and application for diosgenin production by solid-state fermentation.[Pubmed: 29725718]
In this study, endophytic fungi were isolated from Dioscorea zingiberensis C.H. Wright (DZW), and a novel clean process to prepare diosgenin from DZW was developed. A total of 123 strains of endophytic fungi were isolated from different plant tissues of DZW. Among them, the strain Fusarium sp. (CPCC 400709) showed the best activity of hydrolyzing steroidal saponins in DZW into diosgenin. Thus, this strain was used to prepare diosgenin from DZW by solid-state fermentation. The fermentation parameters were optimized using response surface methodology, and a high yield of diosgenin (2.16%) was obtained at 14.5% ammonium sulfate, an inoculum size of 12.3%, and 22 days of fermentation. Furthermore, the highest diosgenin yield (2.79%) was obtained by co-fermentation with Fusarium sp. (CPCC 400709) and Curvularia lunata (CPCC 400737), which was 98.9% of that obtained by β-glucosidase pretreated acid hydrolysis (2.82%). This process is acid-free and wastewater-free, and shows promise as an effective and clean way to prepare diosgenin for use in industrial applications from DZW.
Multiplatform Metabolomics Investigation of Antiadipogenic Effects on 3T3-L1 Adipocytes by a Potent Diarylheptanoid.[Pubmed: 29688022]
Obesity is fast becoming a serious health problem worldwide. Of the many possible antiobesity strategies, one interesting approach focuses on blocking adipocyte differentiation and lipid accumulation to counteract the rise in fat storage. However, there is currently no drug available for the treatment of obesity that works by inhibiting adipocyte differentiation. Here we use a broad-based metabolomics approach to interrogate and better understand metabolic changes that occur during adipocyte differentiation. In particular, we focus on changes induced by the antiadipogenic diarylheptanoid, which was isolated from a traditional Chinese medicine Dioscorea zingiberensis and identified as (3 R,5 R)-3,5-dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-heptane (1). Targeted aqueous metabolic profiling indicated that a total of 14 metabolites involved in the TCA cycle, glycolysis, amino acid metabolism, and purine catabolism participate in regulating energy metabolism, lipogenesis, and lipolysis in adipocyte differentiation and can be modulated by diarylheptanoid 1. As indicated by lipidomics analysis, diarylheptanoid 1 restored the quantity and degree of unsaturation of long-chain free fatty acids and restored the levels of 171 lipids mainly from 10 lipid classes in adipocytes. In addition, carbohydrate metabolism in diarylheptanoid-1-treated adipocytes further demonstrated the delayed differentiation process by flux analysis. Our results provide valuable information for further understanding the metabolic adjustment in adipocytes subjected to diarylheptanoid 1 treatment. Moreover, this study offers new insight into developing antiadipogenic leading compounds based on metabolomics.
Dioscorea zingiberensis C. H. Wright: An overview on its traditional use, phytochemistry, pharmacology, clinical applications, quality control, and toxicity.[Pubmed: 29602601]
Dioscorea zingiberensis C. H. Wright (D. zingiberensis), Dioscoreaceae, is used extensively in traditional Chinese medicines. The aim of the current review paper is to give a comprehensive overview of the traditional usage and phytochemistry of the plant. Clinical studies performed and products prepared from the plant and active principles will be mentioned. In addition a review of the taxonomy of the genus Dioscorea is given.
Comparative Transcriptome Analysis Identifies Putative Genes Involved in Dioscin Biosynthesis in Dioscorea zingiberensis.[Pubmed: 29463020]
Dioscorea zingiberensis is a perennial herb native to China. The rhizome of D. zingiberensis has long been used as a traditional Chinese medicine to treat rheumatic arthritis. Dioscin is the major bioactive ingredient conferring the medicinal property described in Chinese pharmacopoeia. Several previous studies have suggested cholesterol as the intermediate to the biosynthesis of dioscin, however, the biosynthetic steps to dioscin after cholesterol remain unknown. In this study, a comprehensive D. zingiberensis transcriptome derived from its leaf and rhizome was constructed. Based on the annotation using various public databases, all possible enzymes in the biosynthetic steps to cholesterol were identified. In the late steps beyond cholesterol, cholesterol undergoes site-specific oxidation by cytochrome P450s (CYPs) and glycosylation by UDP-glycosyltransferases (UGTs) to yield dioscin. From the D. zingiberensis transcriptome, a total of 485 unigenes were annotated as CYPs and 195 unigenes with a sequence length above 1000 bp were annotated as UGTs. Transcriptomic comparison revealed 165 CYP annotated unigenes correlating to dioscin biosynthesis in the plant. Further phylogenetic analysis suggested that among those CYP candidates four of them would be the most likely candidates involved in the biosynthetic steps from cholesterol to dioscin. Additionally, from the UGT annotated unigenes, six of them were annotated as 3-O-UGTs and two of them were annotated as rhamnosyltransferases, which consisted of potential UGT candidates involved in dioscin biosynthesis. To further explore the function of the UGT candidates, two 3-O-UGT candidates, named Dz3GT1 and Dz3GT2, were cloned and functionally characterized. Both Dz3GT1 and Dz3GT2 were able to catalyze a C3-glucosylation activity on diosgenin. In conclusion, this study will facilitate our understanding of dioscin biosynthesis pathway and provides a basis for further mining the genes involved in dioscin biosynthesis.
Dihydrodiosgenin protects against experimental acute pancreatitis and associated lung injury through mitochondrial protection and PI3Kγ/Akt inhibition.[Pubmed: 29457828]
Acute pancreatitis (AP) is a painful and distressing disorder of the exocrine pancreas with no specific treatment. Diosgenyl saponins extracted from from Dioscorea zingiberensis C. H. Wright have been reported to protect against experimental models of AP. Diosgenin, or its derivatives are anti-inflammatory in various conditions. However, the effects of diosgenin and its spiroacetal ring opened analogue, dihydrodiosgenin (Dydio), on AP have not been determined.
Safety investigation on total steroid saponins extracts from Dioscorea zingiberensis C.H. Wright: Sub-acute and chronic toxicity studies on dogs.[Pubmed: 29066336]
None


