Erycibe obtusifolia
Erycibe obtusifolia
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Erycibe obtusifolia
- Cat.No. Product Name CAS Number COA
-
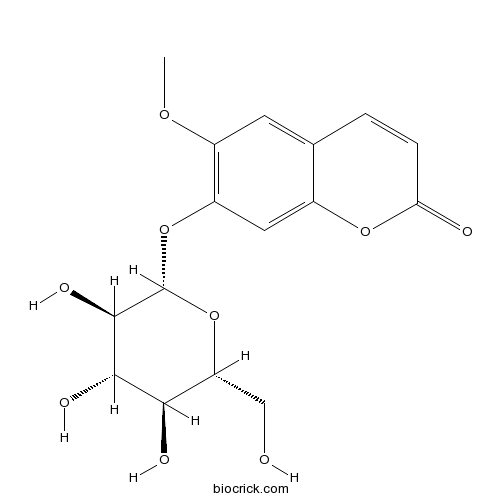
BCN5701
Scopolin531-44-2
Instructions
-
BCN4470
Scopoletin92-61-5
Instructions

[Current research situation of nephrotoxicity of Chinese herbal medicine].[Pubmed: 29600603]
To provide the basis for the future research on the nephrotoxicity of Chinese herbal medicine through systematic and comprehensive summary of all the Chinese herbal medicines which may lead to nephrotoxicity. Foreign resources included PubMed and Cochrane library, and domestic research resources was China Food and Drug Administration(CDFA) Adverse Drug Reaction Monitoring Center database. The databases were searched from establishment to January 1, 2017. There was no limitation on research type. 28 English studies were found, including 97 Chinese herbs or prescriptions with the risk of nephrotoxicity. The following six Chinese herbal medicines with the risk of nephrotoxicity had a large number of studies: aristolochic acid(5 studies), Tripterygium wilfordii(4 studies), Erycibe obtusifolia(2 studies), Rheum palmatum(2 studies), Ephedra sinica(2 studies), and Atractylodes lances(2 studies). The remaining 91 Chinese medicines were reported with risk of nephrotoxicity in only 1 study respectively. CDFA reported 16 Chinese herbal medicines with the risk of nephrotoxicity, including Ganmaoqing Pian(capsule), Zhenju Jiangya Pian, T. wilfordii preparation, Vc-Yinqiao Pian, Chuanhuning injection, Shuanghuanglian injection, Qingkailing injection, Lianbizhi injection, herbal decoction containing Aristolochiae Radix, Guanxin Suhe Wan, Shugan Liqi Wan, Ershiwuwei Songshi Wan, herbal decoction containing Aristolochia Fangchi, herbal granules containing root of Kaempfer Dutchmanspipe, Ganmaotong(tablets), and Longdan Xiegan Wan. Currently, in addition to aristolochic acids, the most reported Chinese herbal medicine with the risk of nephrotoxicity is T. wilfordii preparation.
A Review of Traditional Medicinal Plants from Kachin State, Northern Myanmar.[Pubmed: 27169181]
Medicinal plants are a vital source of medication in developing countries. In Kachin State, Northern Myanmar, the people have a long history of the use of traditional plants for medicinal purposes. This article deals with the 25 most used medicinal plants in Kachin State. They are: Drynariafortunei, Tetrastigma serrulatum, Bauhinia championii, Goniothalamus cheliensis, Juglans regia, Houttuynia cordata, Osmanthus fragrans, Pothos chinensis, Tabemaemontana coronaria, Eryngiumfoetidum, Chloranthus spicatus, Peperomia pellucida, Zanthoxylum armatum, Polygonumfagopyrum, Cymbidiumfloribundum, Amomum kravanh, Coscinium fenestratum, Solanum nigrum, Gnetum parvifolium, Desmodium triquetum, Begonia augustinec, Mappianthus iodoides, Erycibe obtusifolia, Schefflera venulosa, Holarrhena antidysenterica. The different traditional applications, the known chemical constituents and medicinal properties are reported for each plant. The efficacy of several of these plants has been supported by some scientific evidence, while other plants have to be submitted to further investigations to prove the beneficial medicinal properties attributed to them.
Acyl quinic acid derivatives from the stems of Erycibe obtusifolia.[Pubmed: 25256062]
Eleven new acyl quinic acid derivatives, 4-O-caffeoyl-3-O-syringoylquinic acid methyl ester (1), 4-O-caffeoyl-3-O-vanilloylquinic acid (2), 4-O-caffeoyl-3-O-vanilloylquinic acid methyl ester (3), 5-O-caffeoyl-3-O-vanilloylquinic acid (4), 5-O-caffeoyl-3-O-vanilloylquinic acid methyl ester (5), 5-O-caffeoyl-3-O-sinapoylquinic acid (6), 5-O-caffeoyl-4-O-vanilloylquinic acid (7), 4-O-(7‴S, 8‴R)-glycosmisoyl-5-O-caffeoylquinic acid methyl ester (8), 4-O-(7‴S, 8‴R)-glycosmisoyl-5-O-caffeoylquinic acid (9), 3-O-(7‴S, 8‴R)-glycosmisoyl-4-O-caffeoylquinic acid (10), and 3-O-(7‴S, 8‴R)-glycosmisoyl-4-O-caffeoylquinic acid methyl ester (11), have been isolated from the stems of Erycibe obtusifolia together with eight known compounds (12-19). Their structures were elucidated on the basis of spectroscopic data analysis (UV, IR, HRESIMS, CD, and 1D and 2D NMR) and chemical evidence. In in vitro assay, compounds 7 and 16-18 exhibited significant neuroprotective effects against rotenone induced PC12 cell damage at 10 μM.
Quick identification of xanthine oxidase inhibitor and antioxidant from Erycibe obtusifolia by a drug discovery platform composed of multiple mass spectrometric platforms and thin-layer chromatography bioautography.[Pubmed: 24895238]
As a final step of the purine metabolism process, xanthine oxidase catalyzes the oxidation of hypoxanthine and xanthine into uric acid. Our research has demonstrated that Erycibe obtusifolia has xanthine oxidase inhibitory properties. The purpose of this paper is to describe a new strategy based on a combination of multiple mass spectrometric platforms and thin-layer chromatography bioautography for effectively screening the xanthine oxidase inhibitory and antioxidant properties of E. obtusifolia. This strategy was accomplished through the following steps. (i) Separate the extract of E. obtusifolia into fractions by an autopurification system controlled by liquid chromatography with mass spectrometry. (ii) Determine the active fractions of E. obtusifolia by thin-layer chromatography bioautography. (iii) Identify the structure of the main active compounds with the information provided by direct analysis in real time mass spectrometry. (iv) Calculate the IC50 value of each compound against xanthine oxidase using high-performance liquid chromatography. Using the caulis of E. obtusifolia as the experimental material, seven target peaks were screened out as xanthine oxidase inhibitors or antioxidants. Our screening strategy allows for rapid analysis of small molecules with almost no sample preparation and can be completed within a week, making it a useful assay to identify unstable compounds and provide the empirical foundation for E. obtusifolia as a natural remedy for gout and oxidative-stress-related diseases.
Chemical constituents from the roots and stems of Erycibe obtusifolia and their in vitro antiviral activity.[Pubmed: 24081686]
Three new quinic acid derivatives, 4-O-caffeoyl-3-O-sinapoylquinic acid methyl ester (1), 5-O-caffeoyl-4-O-syringoylquinic acid methyl ester (2), and 4-O-caffeoyl-3-O-syringoylquinic acid methyl ester (3), as well as four new coumarin glycosides, 7-O-(3-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (12), 7-O-(6-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (13), 7-O-(2-O-sinapoyl-β-D-glucopyranosyl)-6-methoxycoumarin (14), and 7-O-(6-O-syringoyl-β-D-glucopyranosyl)-6-methoxycoumarin (15), together with eight known compounds (4-11) were isolated from the roots and stems of Erycibe obtusifolia. Their structures were elucidated on the basis of spectroscopic analysis and chemical evidence. All the compounds were screened for their in vitro antiviral activity against respiratory syncytial virus with a cytopathic effect reduction assay. Among them, the di-O-caffeoyl quinates 8-11 displayed a potent in vitro anti-respiratory syncytial virus effect.
Comparison of active constituents, acute toxicity, anti-nociceptive and anti-inflammatory activities of Porana sinensis Hemsl., Erycibe obtusifolia Benth. and Erycibe schmidtii Craib.[Pubmed: 24055469]
Erycibe obtusifolia and Erycibe schmidtii, which belong to the same genus as Erycibe, are widely used in traditional medicine for the treatment of joint pain and rheumatoid arthritis (RA). Porana sinensis has become a widely used substitute for Erycibe obtusifolia and Erycibe schmidtii as they have declined in the wild. In the present work, the content of the main active components, the acute toxicity, the anti-nociceptive and anti-inflammatory activities of Porana sinensis, Erycibe obtusifolia and Erycibe schmidtii were compared, and the mechanisms of anti-nociceptive and anti-inflammatory activities were discussed.
Acyl glycosides lignans, coumarins, and terpenes from the stems of Erycibe obtusifolia.[Pubmed: 23524110]
Nine new acyl glycosides, obtusifosides A-I (1-9), and eight known compounds have been isolated from an EtOH extract of the stems of Erycibe obtusifolia. Their structures were elucidated on the basis of a spectroscopic data analysis (NMR, HRESIMS, and CD) and chemical evidence. The hepatoprotective effects of some of the compounds from d-galactosamine-induced cytotoxicity in HL-7702 hepatic cells were evaluated. Compounds 1, 10, 11, 13, 16, and 17 showed significant hepatoprotective activities compared with the positive control bicyclol at concentrations of 1×10(-5)M.
Prevention of FGF-2-induced angiogenesis by scopoletin, a coumarin compound isolated from Erycibe obtusifolia Benth, and its mechanism of action.[Pubmed: 21896339]
Previous work in our laboratory has shown that scopoletin, one of the main bioactive constituents of Erycibe obtusifolia Benth stems, exerts anti-arthritic activity in vivo partly by preventing synovial angiogenesis. The present study was performed to further investigate the anti-angiogenic potential of scopoletin, focusing on the mechanisms of action in vitro. In the aortic ring sprouting assay, scopoletin (10, 30 and 100 μM) significantly inhibited the growth of endothelial sprouts in a concentration-dependent manner. As to human umbilical vein endothelial cells (HUVECs), scopoletin could inhibit their proliferation, migration and tubule formation induced by FGF-2, especially the proliferation. It also remarkably decreased the expression of VEGF at mRNA and protein levels, and the phosphorylations of IKKα and IκB but not Akt, as well as the degradation of IκB caused by FGF-2 in HUVECs. These findings suggest that scopoletin is substantially able to attenuate FGF-2-induced angiogenesis, and it might act by directly preventing the stimulation action of FGF-2 and by indirectly decreasing the production of VEGF. Scopoletin down-regulated the VEGF expression through NF-κB rather than PI-3K/Akt signaling pathway.
Inhibition of vascular endothelial growth factor-induced angiogenesis by scopoletin through interrupting the autophosphorylation of VEGF receptor 2 and its downstream signaling pathways.[Pubmed: 21078410]
Our previous studies revealed that scopoletin, the main bioactive constituent of Erycibe obtusifolia Benth stems, exerted anti-arthritic activity in vivo partly by preventing synovial angiogenesis. Herein we further investigated the anti-angiogenic potential and related mechanisms of this coumarin compound in vivo and in vitro. On chick chorioallantoic membrane (CAM) model, scopoletin (10, 30, 100 nmol/egg) dose-dependently reduced the blood vessels that were quantified by counting the number of blood vessel branch points. In vitro, scopoletin at concentrations above 30 microM obviously inhibited the VEGF-induced tube formation, proliferation and migration of human umbilical vein endothelial cells (HUVECs). Furthermore, scopoletin was shown to block VEGF-induced autophosphorylation of VEGFR2 but not VEGFR1, and down-regulate the following activation of ERK1/2, p38 MAPK and endothelial nitric oxide synthase (eNOS) as well as the production of nitric oxide (NO) in HUVECs. In sum, our findings further support that scopoletin is a candidate of angiogenesis inhibitors, and it functions by interrupting the autophosphorylation of VEGF receptor 2 (VEGFR2) and the downstream signaling pathways.


