Euphorbia humifusa
Euphorbia humifusa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Euphorbia humifusa
- Cat.No. Product Name CAS Number COA
-
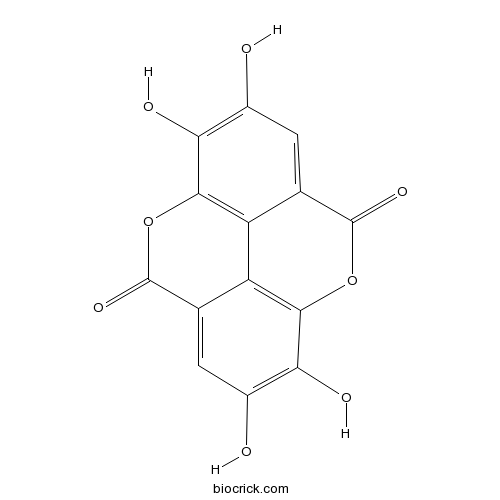
BCN5533
Ellagic acid476-66-4
Instructions
-
BCN5725
Lupeol545-47-1
Instructions

Mechanisms of vasorelaxation induced by total flavonoids of Euphorbia humifusa in rat aorta.[Pubmed: 29151079]
None
Growth inhibitory effect of paratocarpin E, a prenylated chalcone isolated from Euphorbia humifusa Wild., by induction of autophagy and apoptosis in human breast cancer cells.[Pubmed: 27814565]
None
Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression.[Pubmed: 27776550]
Breast cancer is the most common type of malignancy in women worldwide. Euphorbia humifusa Willd (EuH) is a plant that is widely used as a traditional medicine. However, no systemic studies on the anti-cancer effects of EuH have been reported. The aim of this study is to evaluate the anti-metastatic effect of the EuH.
A natural component from Euphorbia humifusa Willd displays novel, broad-spectrum anti-influenza activity by blocking nuclear export of viral ribonucleoprotein.[Pubmed: 26850850]
The need to develop anti-influenza drugs with novel antiviral mechanisms is urgent because of the rapid rate of antigenic mutation and the emergence of drug-resistant viruses. We identified a novel anti-influenza molecule by screening 861 plant-derived natural components using a high-throughput image-based assay that measures inhibition of the influenza virus infection. 1,3,4,6-tetra-O-galloyl-β-D-glucopyranoside (TGBG) from Euphorbia humifusa Willd showed broad-spectrum anti-influenza activity against two seasonal influenza A strains, A/California/07/2009 (H1N1) and A/Perth/16/2009 (H3N2), and seasonal influenza B strain B/Florida/04/2006. We investigated the mode of action of TGBG using neuraminidase activity inhibition and time-of-addition assays, which evaluate the viral release and entry steps, respectively. We found that TGBG exhibits a novel antiviral mechanism that differs from the FDA-approved anti-influenza drugs oseltamivir which inhibits viral release, and amantadine which inhibits viral entry. Immunofluorescence assay demonstrated that TGBG significantly inhibits nuclear export of influenza nucleoproteins (NP) during the early stages of infection causing NP to accumulate in the nucleus. In addition, influenza-induced activation of the Akt signaling pathway was suppressed by TGBG in a dose-dependent manner. These data suggest that a putative mode of action of TGBG involves inhibition of viral ribonucleoprotein (vRNP) export from the nucleus to the cytoplasm consequently disrupting the assembly of progeny virions. In summary, TGBG has potential as novel anti-influenza therapeutic with a novel mechanism of action.
Antifungal Activity of Ellagic Acid In Vitro and In Vivo.[Pubmed: 25919446]
Ellagic acid (EA) has been shown to have antioxidant, antibacterial, and anti-inflammatory activities. In Uighur traditional medicine, Euphorbia humifusa Willd is used to treat fungal diseases, and recent studies suggest that it is the EA content which is responsible for its therapeutic effect. However, the effects of EA on antifungal activity have not yet been reported. This study aimed to investigate the inhibitory effect of EA on fungal strains both in vitro and in vivo. The minimal inhibitory concentration (MIC) was determined by the National Committee for Clinical Laboratory Standards (M38-A and M27-A2) standard method in vitro. EA had a broad spectrum of antifungal activity, with MICs for all the tested dermatophyte strains between 18.75 and 58.33 µg/ml. EA was also active against two Candida strains, with MICs between 25.0 and 75.0 µg/ml. It was inactive against Candida glabrata. The susceptibility of six species of dermatophytes to EA was comparable with that of the commercial antifungal, fluconazole. The most sensitive filamentous species was Trichophyton rubrum (MIC = 18.75 µg/ml). Studies on the mechanism of action using an HPLC-based assay and an enzyme linked immunosorbent assay showed that EA inhibited ergosterol biosynthesis and reduced the activity of sterol 14α-demethylase P450 (CYP51) in the Trichophyton rubrum membrane, respectively. An in vivo test demonstrated that topical administration of EA (4.0 and 8.0 mg/cm(2) ) significantly enhanced the cure rate in a guinea-pig infection model of Trichophyton rubrum. The results suggest that EA has the potential to be developed as a natural antifungal agent.
[Action of Euphorbia humifusa effective fraction on membrane biosynthesis and the gene expression of proteases MEP and SUB of Trichophyton rubrum].[Pubmed: 24761622]
This study is to investigate the effect of Euphorbia humifusa effective fraction (EHEF) on the CYP51 enzyme activity, the lanosterol content and the MEP, SUB gene expression of Trichophyton rubrum. Trichophyton rubrum was treated by EHEF for 7 days at 26 degrees C. The activity of CYP51 enzyme of Trichophyton rubrum in the cell membrane was determined by using ELISA kit, and the lanosterol content was investigated by using high performance liquid chromatography (HPLC), and the MEP, SUB gene expression of Trichophyton rubrum was detected with the reverse transcription polymerase chain reaction (RT-PCR) method. Results showed that EHEF can decrease the membrane CYP51 enzyme activity, and it also can accumulate the fungal lanosterol in a dose-dependent manner, and it also can decrease the gene expression of MEP and SUB. The antifungal mechanism of EHEF may be related to the inhibition on CYP51 enzyme activity, and to the effects on fungal cell membrane ergosterol biosynthesis. It may also play an antifungal effect by inhibiting the MEP, SUB gene expression of fungal proteases.
Anti-inflammatory components of Euphorbia humifusa Willd.[Pubmed: 24679441]
Two new compounds, euphorbinoside (1) and dehydropicrorhiza acid methyl diester (2), along with 24 known compounds (3-26) were isolated from Euphorbia humifusa Willd. The effects of these compounds on soluble epoxide hydrolase (sEH) inhibitory activity were evaluated. Flavonoid compounds (10-21) exhibited high sEH inhibitory activity. Among them, compounds 12, 13, and 19 greatly inhibited sEH enzymatic activity, with IC50 values as low as 18.05±1.17, 18.64±1.83, and 17.23±0.84 μM, respectively. In addition, the effects of these compounds on lipopolysaccharide (LPS)-induced nitric oxide (NO) and tumor necrosis factor alpha (TNF-α) production by RAW 264.7 cells were investigated. Compounds 3-6, 8, 18, 20-23, and 25-26 inhibited the production of both NO and TNF-α, with IC50 values ranging from 11.1±0.9 to 45.3±1.6 μM and 14.4±0.5 to 44.5±1.2 μM, respectively.


