Ganoderma theaecolum
Ganoderma theaecolum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Ganoderma theaecolum
- Cat.No. Product Name CAS Number COA
-
BCN5859
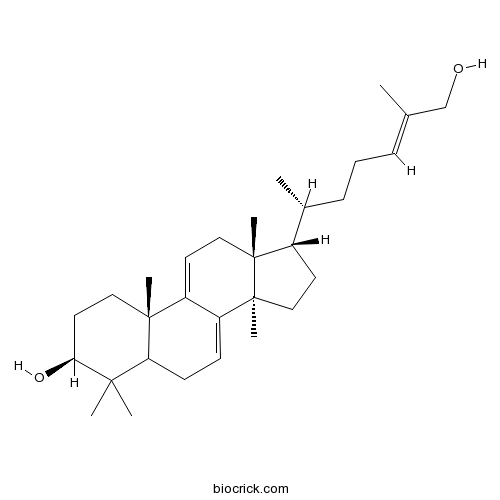
Ganoderol B104700-96-1
Instructions
Meroterpenoids from the fruiting bodies of Ganoderma theaecolum.[Pubmed: 29378219]
None
Three new triterpenoids from Ganoderma theaecolum.[Pubmed: 28152606]
None
Ganotheaecolin A, a Neurotrophic Conjugated Ergosterol with a Naphtho[1,8-ef]azulene Scaffold from Ganoderma theaecolum.[Pubmed: 28124916]
Ganotheaecolin A (1), a novel ergosterol with a rare naphtho[1,8-ef]azulene ring system, was isolated from the fruiting bodies of Ganoderma theaecolum. Its structure was determined by spectroscopic data and computational methods. Compound 1 represents a 6/6/7/5-fused carbon skeletal ergosterol typically formed by Wagner-Meerwein rearrangement, whose plausible biosynthetic pathway was briefly discussed. Finally, the neurotrophic activity of 1 was examined using PC12 cells.
[Triterpenoids from Ganoderma theaecolum].[Pubmed: 28875673]
Fifteenlanostane triterpenoids were isolated from the ethanol extract of Ganoderma theaecolum by means of preparative HPLC,column chromatography over silica gel,ODS and were identified as lucidone C(1),lucidone D(2),7-oxo-ganoderic acid Z2(3),7-oxo-ganoderic acid Z(4),ganoderenicacid H(5),ganoderenic acid B(6),3β,7β-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid(7),3β,7β-dihydroyl-11,15,23-trioxo-lanost-8,16-dien-26-oic acid methyl ester(8),ganolucidic acid B(9),ganolucidate F(10),methyl ganoderate C2(11),ganoderic acid ζ(12),ganoderic acid AP3(13),methyl ganoderate B(14),and ganoderol B(15). Compounds 1-15 were isolated from this specie for the first time.
Secondary metabolites from Ganoderma.[Pubmed: 25975187]
Ganoderma is a genus of medicinal mushrooms. This review deals with secondary metabolites isolated from Ganoderma and their biological significance. Phytochemical studies over the last 40years led to the isolation of 431 secondary metabolites from various Ganoderma species. The major secondary compounds isolated are (a) C30 lanostanes (ganoderic acids), (b) C30 lanostanes (aldehydes, alcohols, esters, glycosides, lactones, ketones), (c) C27 lanostanes (lucidenic acids), (d) C27 lanostanes (alcohols, lactones, esters), (e) C24, C25 lanostanes (f) C30 pentacyclic triterpenes, (g) meroterpenoids, (h) farnesyl hydroquinones (meroterpenoids), (i) C15 sesquiterpenoids, (j) steroids, (k) alkaloids, (l) prenyl hydroquinone (m) benzofurans, (n) benzopyran-4-one derivatives and (o) benzenoid derivatives. Ganoderma lucidum is the species extensively studied for its secondary metabolites and biological activities. Ganoderma applanatum, Ganoderma colossum, Ganoderma sinense, Ganoderma cochlear, Ganoderma tsugae, Ganoderma amboinense, Ganoderma orbiforme, Ganoderma resinaceum, Ganoderma hainanense, Ganoderma concinna, Ganoderma pfeifferi, Ganoderma neo-japonicum, Ganoderma tropicum, Ganoderma australe, Ganoderma carnosum, Ganoderma fornicatum, Ganoderma lipsiense (synonym G. applanatum), Ganoderma mastoporum, Ganoderma theaecolum, Ganoderma boninense, Ganoderma capense and Ganoderma annulare are the other Ganoderma species subjected to phytochemical studies. Further phytochemical studies on Ganoderma could lead to the discovery of hitherto unknown biologically active secondary metabolites.
Triterpenoids of Ganoderma theaecolum and their hepatoprotective activities.[Pubmed: 25111010]
Five new lanostane triterpenoids, ganoderic acid XL1 (1), ganoderic acid XL2 (2), 20-hydroxy-ganoderic acid AM1 (3), ganoderenic acid AM1 (4) and ganoderesin C (5), together with five known triterpenoids (6-10) were isolated from the fruiting bodies of Ganoderma theaecolum. Chemical structures were elucidated on the basis of spectroscopic evidence, including 1D, 2D NMR, mass spectrometric data and circular dichroism spectra. Compounds 1, 4, 5, 8, 9 and 10 (10 μM) exhibited hepatoprotective activities against DL-galactosamine-induced cell damage in HL-7702 cells.


