Gladiolus gandavensis
Gladiolus gandavensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Gladiolus gandavensis
- Cat.No. Product Name CAS Number COA
-
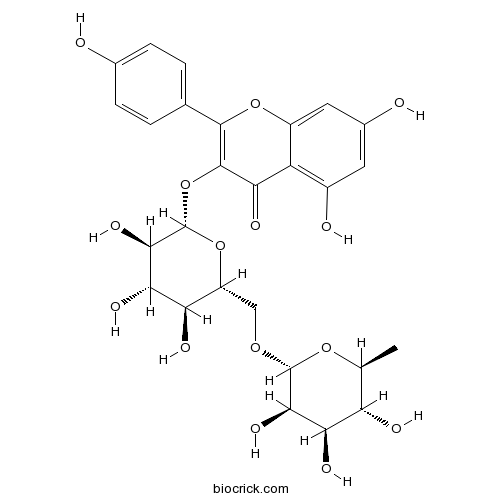
BCN1126
Nicotiflorin17650-84-9
Instructions
[Studies on the chemical constituents from the aerial parts of Gladiolus gandavensis].[Pubmed: 21213537]
To study the chemical constituents of the aerial parts of Gladiolus gandavensis.
Anthraquinones from Gladiolus gandavensis.[Pubmed: 15621627]
Five new anthraquinones have been obtained from the ethanolic extracts of the subterranean corms of Gladiolus gandavensis Van Houtt. Their structures were elucidated as 3,8-dihydroxy-6-methoxy-1-methyl-anthraquinone (gandavensin D, 1), methyl 3,8-dihydroxy-6,7-methylenedioxy-1-methyl-anthraquinone- 2-carboxylate (gandavensin E, 2), 2,3,8-trihydroxy-6-methoxy-1-methoxymethyl-anthraquinone (gandavensin F, 3), 8-hydroxy-3,6-dimethoxy-1-methyl-anthraquinone-2-carboxylic acid (gandavensin G, 4) and 1,7-dihydroxy-3,6-dimethoxy-anthraquinone (gandavensin H, 5) on the basis of spectral data. The known compounds isolated for the first time from this plant have been determined to be methyl 3,6,8-trihydroxy-7-methoxy-1-methyl-anthraquinone-2-carboxylate (6), methyl 3,6,8-trihydroxy-1-methyl-anthraquinone-2-carboxylate (7), 3,8-dihydroxy-6-methoxy-1-methyl-anthraquinone-2-carboxylic acid (8), 3,6,8-trihydroxy-1-methyl-anthraquinone-2-carboxylic acid (9), 6,8-dihydroxy-3-methoxy-1-methyl-anthraquinone-2-carboxylic acid (10), methyl 3,8-dihydroxy-6-methoxy-1-methyl-anthraquinone-2-carboxylate (11) and methyl 3,7,8-trihydroxy-1-methyl-anthraquinone-2-carboxylate (12).
Molecular characterization of a pathogenesis-related protein 8 gene encoding a class III chitinase in rice.[Pubmed: 15055541]
A cDNA encoding a class III chitinase (Oschib1) was isolated from a cDNA library constructed from rice leaves infected with the blast fungus Magnaporthe grisea. The cDNA contains an open reading frame of 861 nucleotides encoding 286 amino acid residues with a pI of 5.06. The deduced amino acid sequence of Oschibl has a high level of similarity with class IIIb chitinases of Gladiolus gandavensis (46%) and Tulipa bakeri (49%). A high level of Oschibl mRNA was detected after inoculation with M. grisea or Xanthomonas oryzae pv. oryzae. Expression of Oschib1 was induced more rapidly when an avirulent strain of M. grisea was inoculated (incompatible interaction) than when a virulent strain was used (compatible interaction). Expression of Oschibl was also induced by treatment of signaling molecules such as salicylic acid, ethylene, and methyl jasmonic acid, and by treatment with H2O2 or CuSO4. The induction patterns of Oschibl expression suggest that Oschib1 may be involved in defense response against pathogen infections and may be classified as a member of pathogenesis-related protein 8 in rice.
New anthraquinones from Gladiolus gandavensis.[Pubmed: 14604240]
Two new anthraquinones, methyl 8-hydroxy-3-methoxy-6,7-methylenedioxy-1-methylanthraquinone-2-carboxylate (gandavensin A, 1) and methyl 8-hydroxy-3,6,7-trimethoxy-1-methylanthraquinone-2-carboxylate (gandavensin B, 2), have been isolated from the light petroleum extract of the subterranean corm of Gladiolus gandavensis Van Houtt., along with methyl 8-hydroxy-3,6-dimethoxy-1-methylanthraquinone-2-carboxylate (3), methyl trans-p-methoxycinnamate (4), 5,7-dimethoxy-2-methylchromone (5), and 5-hydroxy-2-hydroxymethyl-7-methoxychromone (6). Their structures were elucidated on the basis of spectral data.
A new anthraquinone from Gladiolus gandavensis.[Pubmed: 14526918]
A new anthraquinone, 1,6,7-trihydroxy-3-methoxyanthraquinone, along with three known compounds methyl trans-p-hydroxycinnamate, eugenin and 1,3,6-trihydroxy-8-methylanthraquinone, were isolated from the subterranean rhizomes of Gladiolus gandavensis Van Houtt. Their structures were established on the basis of spectroscopic data including 2D NMR techniques and chemical methods.
Complete amino acid sequence of chitinase-a from bulbs of gladiolus (Gladiolus gandavensis).[Pubmed: 9532802]
The complete amino acid sequence of gladiolus bulb chitinase-a (GBC-a) was determined. First the tryptic peptides from GBC-a after it was reduced and S-carboxymethylated were sequenced and then the peptides were further studied by chemical cleavage of the enzyme. GBC-a consisted of 274 amino acid residues and had a molecular mass of 30,714 Da. Two consensus sequences essential for chitinase activity by plant class III chitinases were conserved in GBC-a, although its sequence similarity with plant class III chitinases was less than 20%. Sequence comparison of GBC-a with sequences of other proteins in a protein identification resource (PIR) showed that the GBC-a sequence was 33% similar to that of narbonin, a seed storage 2S globulin from narbon beans.
Purification and characterization of two chitinase isoforms from the bulbs of gladiolus (Gladiolus gandavensis).[Pubmed: 9438997]
Two chitinase isoforms, designated GBC-a and GBC-b, were purified from the bulbs of gladiolus (Gladiolus gandavensis) using CM-cellulose column chromatography followed by Butyl-Toyopearl 650 M hydrophobic column chromatography, gel filtration on Sephadex G-75, and Mono-S FPLC. GBC-a and GBC-b are weakly acidic and weakly basic proteins with molecular masses of 30 kDa, and isoelectric points of 6.0 and 7.5, respectively. GBC-a and GBC-b were found to be homologous proteins with similar amino acid compositions and N-terminal sequences. The number of half-cystine residues in GBC-a and GBC-b was only one each, which is much lower than those of plant class I (15-17 Cys residues/mol), class II (5-8 Cys residues/mol), and class III (6 Cys residues/mol) chitinases. The N-terminal sequences of GBC-a and GBC-b were completely different from those of plant three classes of chitinases. The optimal pHs of these chitinases toward glycolchitin were pH 5. GBC-a hydrolyzed (GlcNAc)5 into (GlcNAc)2, (GlcNAc)3 and (GlcNAc)4, and (GlcNAc)5 into (GlcNAc)2 and (GlcNAc)3.
Isolation of Sperms from the Pollen Tubes of Flowering Plants during Fertilization.[Pubmed: 16666200]
Sperm cells have been isolated from pollen tubes growing in style segments of the dicotlyledon Rhododendron macgregoriae and the monocotyledon Gladiolus gandavensis by the in vivo/in vitro method at various stages of fertilization. Pollen tubes emerged from the cut end of the style into agar medium, and more than 95% contained sperm cells. Sperm cells were released from the pollen tubes by osmotic shock or by placing styles in wall-degrading enzymes: 0.5% macerozyme and 1% cellulase. The isolated sperms were ellipsoidal protoplasts of diameter about 2 x 3 micrometers in Gladiolus and about 3 x 4 micrometers in Rhododendron. After isolation, a proportion of the sperm cells occurred in pairs linked at one end by finger-like connections. The pairs of isolated sperms were dimorphic in terms of surface area and volume. By cutting the styles at various positions and times after pollination, the potential exists to detect changes in sperm gene expression associated with fertilization.


