Goniothalamus cheliensis
Goniothalamus cheliensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Goniothalamus cheliensis
- Cat.No. Product Name CAS Number COA
-
BCN4537
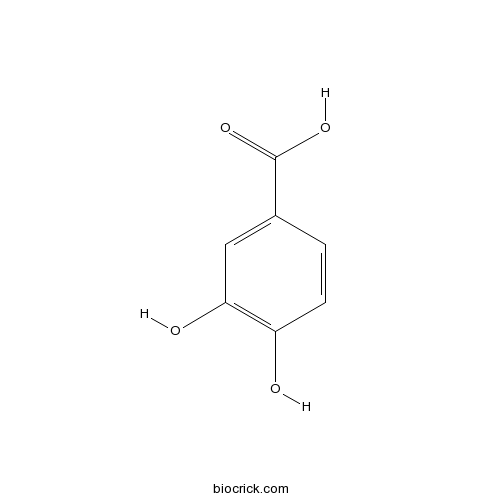
3,4-Dihydroxybenzoic acid99-50-3
Instructions
Cheliensisin A (Chel A) induces apoptosis in human bladder cancer cells by promoting PHLPP2 protein degradation.[Pubmed: 27556506]
Cheliensisin A (Chel A), a styryl-lactone compound extracted from Goniothalamus cheliensis, is reported to have significant anti-cancer effects in various cancer cells. Here we demonstrated that Chel A treatment resulted in apoptosis and an inhibition of anchorage-independent growth in human bladder cancer T24, T24T and U5637 cells. Mechanistic studies showed that such effect is mediated by PH domain and Leucine rich repeat Protein Phosphatases (PHLPP2) protein. Chel A treatment led to PHLPP2 degradation and subsequently increased in c-Jun phosphorylation. Moreover PHLPP2 degradation could be attenuated by inhibition of autophagy, which was mediated by Beclin 1. Collectively, we discover that Chel A treatment induces Beclin-dependent autophagy, consequently mediates PHLPP2 degradation and JNK/C-Jun phosphorylation and activation, further in turn contributing to apoptosis in human bladder cancer cells. Current studies provide a significant insight into understanding of anticancer effect of Chel A in treatment of human bladder cancer.
A Review of Traditional Medicinal Plants from Kachin State, Northern Myanmar.[Pubmed: 27169181]
Medicinal plants are a vital source of medication in developing countries. In Kachin State, Northern Myanmar, the people have a long history of the use of traditional plants for medicinal purposes. This article deals with the 25 most used medicinal plants in Kachin State. They are: Drynariafortunei, Tetrastigma serrulatum, Bauhinia championii, Goniothalamus cheliensis, Juglans regia, Houttuynia cordata, Osmanthus fragrans, Pothos chinensis, Tabemaemontana coronaria, Eryngiumfoetidum, Chloranthus spicatus, Peperomia pellucida, Zanthoxylum armatum, Polygonumfagopyrum, Cymbidiumfloribundum, Amomum kravanh, Coscinium fenestratum, Solanum nigrum, Gnetum parvifolium, Desmodium triquetum, Begonia augustinec, Mappianthus iodoides, Erycibe obtusifolia, Schefflera venulosa, Holarrhena antidysenterica. The different traditional applications, the known chemical constituents and medicinal properties are reported for each plant. The efficacy of several of these plants has been supported by some scientific evidence, while other plants have to be submitted to further investigations to prove the beneficial medicinal properties attributed to them.
Goniolactone C, a styryl lactone derivative, inhibits PDGF-BB-induced vascular smooth muscle cell migration and proliferation via PDGFR/ERK signaling.[Pubmed: 25432005]
Platelet-derived growth factor-BB (PDGF-BB) and its downstream effector, extracellular signal-regulated kinase 1/2 (ERK1/2) MAP kinase, initiate a multitude of biological effects, including vascular smooth muscle cell (VSMC) proliferation and migration, which are critical events in the initiation and development of restenosis following percutaneous transluminal coronary angioplasty (PTCA). Styryl lactones are natural products that have been demonstrated to possess anti-proliferative activities. Goniolactone C is a styryl lactone derivative that was first extracted from Goniothalamus cheliensis Hu. In the present study, we investigated the effects of goniolactone C on VSMC migration and proliferation. We found that goniolactone C preferentially interacted with cellular systems that rely on PDGF signaling but not those that rely on epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) signaling. Goniolactone C strongly inhibited PDGF-BB-induced VSMC migration and proliferation. goniolactone C-mediated inhibition of VSMC proliferation was associated with cell cycle arrest, while goniolactone C-mediated inhibition of VSMC migration was associated with the suppression of adhesion molecule expression. In addition, goniolactone C directly inhibited PDGFR-β kinase activity, thereby blocking the downstream effector of PDGF-BB. Thus, the results of the present study suggest a novel adjunctive pharmacological strategy that may be used to prevent angioplasty-related restenosis.
Crucial role of c-Jun phosphorylation at Ser63/73 mediated by PHLPP protein degradation in the cheliensisin a inhibition of cell transformation.[Pubmed: 25281487]
Cheliensisin A (Chel A), as a novel styryl-lactone isolated from Goniothalamus cheliensis Hu, has been demonstrated to have an inhibition of EGF-induced Cl41 cell transformation via stabilizing p53 protein in a Chk1-dependent manner, suggesting its chemopreventive activity in our previous studies. However, its underlying molecular mechanisms have not been fully characterized yet. In the current study, we found that Chel A treatment could increase c-Jun protein phosphorylation and activation, whereas the inhibition of c-Jun phosphorylation, by ectopic expression of a dominant-negative mutant of c-Jun, TAM67, reversed the Chel A inhibition of EGF-induced cell transformation and impaired Chel A induction of p53 protein and apoptosis. Moreover, our results indicated that Chel A treatment led to a PHLPP downregulation by promoting PHLPP protein degradation. We also found that PHLPP could interact with and bind to c-Jun protein, whereas ectopic PHLPP expression blocked c-Jun activation, p53 protein and apoptotic induction by Chel A, and further reversed the Chel A inhibition of EGF-induced cell transformation. With the findings, we have demonstrated that Chel A treatment promotes a PHLPP protein degradation, which can bind to c-Jun and mediates c-Jun phosphorylation, and further leading to p53 protein induction, apoptotic responses, subsequently resulting in cell transformation inhibition and chemopreventive activity of Chel A.
Hydrogen peroxide/ATR-Chk2 activation mediates p53 protein stabilization and anti-cancer activity of cheliensisin A in human cancer cells.[Pubmed: 24553354]
Chiliensisine A (Chel A) as a novel styryl-lactone isolated from Goniothalamus cheliensis Hu has been indicated to be a chemotherapeutic agent in Leukemia HL-60 cells. However, its potential for cancer treatment and the underlying mechanisms are not deeply investigated to the best of our knowledge. Current studies showed that Chel A could trigger p53-mediated apoptosis, accompanied with dramatically inhibition of anchorage-independent growth of human colon cancer HCT116 cells. Further studies found that Chel A treatment resulted in p53 protein stabilization and accumulation via the induction of its phosphorylation at Ser20 and Ser15. Moreover, Chel A-induced p53 protein accumulation and activation required ATR/Chk2 axis, which is distinct from the mechanism that we have most recently identified the Chk1/p53-dependent apoptotic response by Chel A in normal mouse epidermal Cl41 cells. In addition, our results demonstrated that hydrogen peroxide generation induced by Chel A acted as a precursor for all these signaling events and downstream biological effects. Taken together, we have identified the Chel A as a new therapeutic agent, which highlights its potential for cancer therapeutic effect.
Cheliensisin A inhibits EGF-induced cell transformation with stabilization of p53 protein via a hydrogen peroxide/Chk1-dependent axis.[Pubmed: 23852422]
Cheliensisin A (Chel A), a novel styryl-lactone isolated from Goniothalamus cheliensis Hu, has been shown to induce apoptosis in human promyelocytic leukemia HL-60 cells with Bcl-2 downregulation. Yet, the potential chemopreventive effect of Chel A has not been explored. Here, we showed that Chel A treatment with various concentrations (0.5, 1.0, 2.0, and 4.0 μmol/L) for 3 weeks could dramatically inhibit EGF-induced cell transformation in Cl41 cells (IC50 ∼2.0 μmol/L). Also, coincubation of Cl41 cells with Chel A (2.0 and 4.0 μmol/L) for 48 hours could induce cell apoptosis in a caspase-3-dependent manner. Mechanically, Chel A treatment could result in increased p53 phosphorylation at Ser15 and elevated p53 total protein expression. Moreover, we found that p53 induction by Chel A was regulated at the protein degradation level, but not at either the transcription or the mRNA level. Further studies showed that p53 stabilization by Chel A was mediated via induction of phosphorylation and activation of Chk1 protein at Ser345. This notion was substantiated by the results that transfection of dominant negative mutant of Chk1 (GFP-Chk1 D130A) significantly attenuated the p53 protein expression, cell apoptosis, and inhibition of cell transformation by Chel A. Finally, increased hydrogen peroxide was found to mediate Chk1 phosphorylation at Ser345, p53 protein induction, cell apoptotic induction, and transformation inhibition following Chel A treatment. Taken together, our studies identify Chel A as a chemopreventive agent with the understanding of the molecular mechanisms involved.
[Analysis of styryllactones from Goniothalamus cheliensis by UPLC-Q-TOF-MS].[Pubmed: 21837975]
To analyze the styryllactone components in Goniothalamus cheliensis Hu (Annonaceae).
Development of novel conformation-constrained cytotoxic derivatives of cheliensisin A by embedment of small heterocycles.[Pubmed: 21775031]
Cheliensisin A is a natural styryl-lactone isolated from Goniothalamus cheliensis Hu in considerably large quantity with putative anticancer activities. However, its poor water solubility and chemical instability have precluded cheliensisin A as a potential drug candidate. To explore the strategy to overcome these problems, 21 novel derivatives of cheliensisin A with different substitutions at C-7 and C-8 positions were designed and synthesized. Inhibition of proliferation against five tumors cell lines indicates that eight new derivatives with embedment of oxazole or oxazoline exhibit improved cytotoxicity on SK-BR-3 and PANC-1, and compounds 2d and 2g show 5-8 folds higher potency than cisplatin. HPLC investigation of representative compounds indicates that oxazole and oxazoline analogs exhibit much improved chemical stability than their natural parent.
Three new bis-styryllactones from Goniothalamus cheliensis.[Pubmed: 21075179]
Three new bis-styryllactone analogues, goniolactones G-I (1-3), have been isolated from the root barks of Goniothalamus cheliensis. Their structures were established on the basis of extensive spectroscopic investigation including HR-ESI-MS and 2D NMR (HSQC, HMBC, (1)H-(1)H COSY, ROESY).
Induction of leukemia cell apoptosis by cheliensisin A involves down-regulation of Bcl-2 expression.[Pubmed: 15842784]
To investigate the apoptosis-inducing effect of cheliensisin A (GC-51), a novel styryl-lactone isolated from Goniothalamus cheliensis, on human promyelocytic leukemia HL-60 cells and the mechanism of action involved.


