Helichrysum arenarium
Helichrysum arenarium
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Helichrysum arenarium
- Cat.No. Product Name CAS Number COA
-
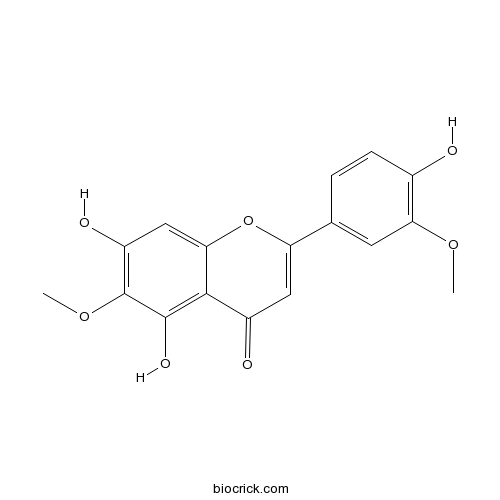
BCN2529
Jaceosidin18085-97-7
Instructions
-
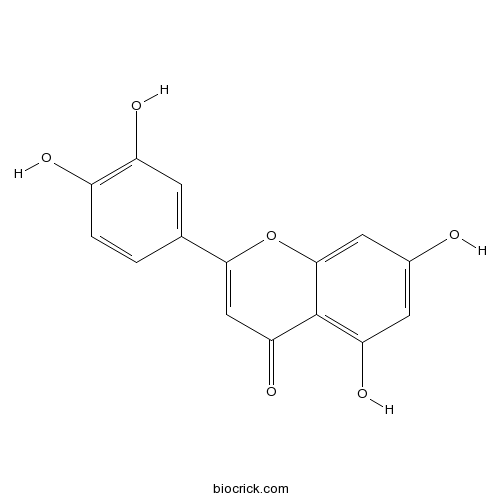
BCN5600
Luteolin491-70-3
Instructions
Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers.[Pubmed: 29438342]
None
Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. MOENCH through the pathway of anti-inflammation.[Pubmed: 28479197]
We have successfully established AS model using thoracic aortas vascular ring which evaluated by the morphological changes of blood vessels, the proliferation of VSMC, and the expression of inflammation factors VEGF, CRP, JNK2 and p38. This AS model has the advantages of low cost, convenient and short period of established time. Moreover, we investigated the anti-AS activities of 7 flavonoids Narirutin (1), Naringin (2), Eriodictyol (3), Luteolin (4), Galuteolin (5), Astragalin (6), Kaempferol (7) from flowers of Helichrysum arenarium L. MOENCH by examining the vascular morphology, the inhibition on the expression of inflammation factors CRP, VEGF, JNK2, p38. In addition, we investigated the anti-AS activities of these 7 flavonoids by examining NO secretion of RAW264.7 cells in response to LPS. All above inflammation factors have been proved to be involved in the formation of AS. After comprehensive analysis of all results to discuss the structure-activity relationship, we summarized the conclusions at follow: compounds 1-7 could inhibit the expression of VEGF, CRP, JNK2, p38 and NO at different level, and we evaluated that flavonol aglycone have more significant anti-inflammation than it's glycoside, and the anti-AS activity of flavonols were stronger than flavanones and flavones, which means that 3-group might be the effective group. Eventually, we supposed the main anti-inflammatory mechanism of these compounds was to reduce the expression of CRP, inhibit the kinases activity of JNK2 and p38, and then the MAPK pathway was suppressed, which resulted in the decrease of NO synthesis, VEGF expression and endothelial adhesion factor expression. And eventually, the scar tissue and vascular stenosis formations were prevented. This conclusion suggested flavonoids have the potential of preventing AS formation.
ELECTROCHEMICAL FINGERPRINT STUDIES OF SELECTED MEDICINAL PLANTS RICH IN FLAVONOIDS.[Pubmed: 26647621]
The combination of a size-exclusion column (SEC) with electrochemical (voltammetric) detection at a boron-doped diamond electrode (BDDE) was applied for studying the correlations between electroactive Cu and Fe species with phenolic groups of flavonoids. For comparison with electrochemical results, SEC-HPLC-DAD detection was used. The studied plant material comprised of: Betula verrucosa Ehrh., Equisetun arvense L., Polygonum aviculare L., Viola tricolor L., Crataegus oxyacantha L., Sambucus nigra L. and Helichrysum arenarium (L.) Moench. Based upon the results, high negative correlation was found for the chromatographic peak currents at 45 min with the sum of Cu and Fe for the aqueous extracts of Sambucus, Crataegus and Betula species, and for the peak currents at 65 min of the aqueous extracts of Sambucus, Crataegus, Helichrysum and Betula botanical species. This behavior confirms that it is mainly the flavonoids with easily oxidizable phenolic groups which are strongly influenced by the presence of Cu and Fe. Moreover, the electrochemical profiles obtained thanks to the use of HPLC hyphenated with voltammetric detection can be potentially applied for fingerprint studies of the plant materials used in medicine.
[CONTENT OF OXIDATIVE STRESS MARKERS IN BLOOD PLASMA UNDER THE ACTION OF EXTRACTS OF GRATIOLA OFFICINALIS L., HELICHRYSUM ARENARIUM (L.) MOENCH, AND ANTHOCYANIN FORMS OF ZEA MAYS L].[Pubmed: 26591206]
The effect of aqueous solutions of dry ethanol extracts of Gratiola officinalis L., Helichrysum arenarium (L.) Moench, and anthocyanin forms of Zea mays L. on the dioxidin-induced lipid peroxidation in blood has been studied on rats. It is established that all these extracts are capable of reducing the amount of avera- ge-mass (AM) molecules and malonic dialdehyde (MDA) in rat blood plasma. The extract of Gratiola officinalis L. reduces the concentration of AM and MDA moleules by 43%. The extract of Helichrysum arenarium (L.) Moench reduces the concentration of AM molecules on the average by 18.66% (within 9.22 -34.81%) and MDA by 49.36% (within 34.12-79.75%). The Extract of anthocyanin forms of Zea mays L. does not reduce the concentration of AM mo- lecules, but reduces the amount of MDA in the blood of rats on average by 27.88% (within 21.58-37.82%) (p < 0.01).
[THE EFFECT OF PLANT EXTRACTS ON THE CYCLOPHOSPHAMIDE INDUCTION OF MICRONUCLEUS IN RED BLOOD CELLS OF OUTBRED WHITE MICE].[Pubmed: 26495712]
Antimutagenic properties flavonoids extracts of the three plants Gratiola officinalis L., Helichrysum arenarium L., anthocyanin forms Zea mays L. were investigated. Analysis was performed by counting the micronucleus in peripheral blood erythrocytes outbred white mice; the mutagen was cyclophosphamide. Selected extracts reduce the number of micronucleus. Gratiola officinalis L. extract reduces the mutagenic action of cyclo- phosphamide at a dose of 200 mg/kg, Helichrysum arenarium L. extract--at doses of 100 and 200 mg/kg (maximum protective effect was observed at a dose of 200 mg/kg), anthocyanin forms Zea mays L. extract at doses of 50, 100 and 200 mg/kg (a dose of 50 mg/kg--maximum antimutagenic effect).
Revision of Heringina Aczél, 1940 (Diptera: Tephritidae), with description of a new species from Iran and Turkey.[Pubmed: 25947794]
The genus Heringina Aczél, 1940 is revised and shown to belong to the Tephritis group of genera and is closely related to Tephritis and Multireticula. Literature records are revised, and available collection material is listed. The genus includes two species: H. guttata (Fallén 1814) originally described from the sand dunes of southern Sweden and occurring from the Baltic region through Ukraine and Caucasus to Turkey, Iran, Kazakhstan, and Kyrgyzstan, and Heringina arezoana sp. nov., found in Iran and eastern Turkey. Both species are described, illustrated, and keyed. Host plants and localization of larvae remain unknown; adult flies of both species are commonly swept from (but never reared) flower heads of Helichrysum arenarium. Other records of host plants listed by Boie (1847) and repeated in most important European monographs, are obviously based on misidentified flies. Possible relationships of Heringina with Tephritis and Multireticula are discussed.
Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium.[Pubmed: 25921859]
A methanol extract of everlasting flowers of Helichrysum arenarium L. Moench (Asteraceae) was found to inhibit the increase in blood glucose elevation in sucrose-loaded mice at 500 mg/kg p.o. The methanol extract also inhibited the enzymatic activity against dipeptidyl peptidase-IV (DPP-IV, IC50 = 41.2 μg/ml), but did not show intestinal α-glucosidase inhibitory activities. From the extract, three new dimeric dihydrochalcone glycosides, arenariumosides V-VII (2-4), were isolated, and the stereostructures were elucidated based on their spectroscopic properties and chemical evidence. Of the constituents, several flavonoid constituents, including 2-4, were isolated, and these isolated constituents were investigated for their DPP-IV inhibitory effects. Among them, chalconaringenin 2'-O-β-D-glucopyranoside (16, IC50 = 23.1 μM) and aureusidin 6-O-β-D-glucopyranoside (35, 24.3 μM) showed relatively strong inhibitory activities.


