Homalomena occulta
Homalomena occulta
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Homalomena occulta
- Cat.No. Product Name CAS Number COA
-
BCN1664
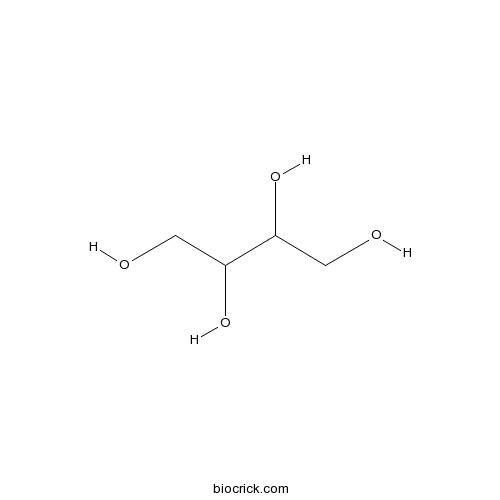
Erythritol149-32-6
Instructions
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Sesquiterpenoids from the Rhizomes of Homalomena occulta.[Pubmed: 27379497]
Naturally occurring oplopanane sesquiterpenoids are rarely reported. A phytochemical investigation on the rhizomes of Homalomena occulta (Lours) has resulted in the discovery of six oplopanane sesquiterpenoids (1-6), including four new (1-4) and one 3,5-seco-oplopanane (6), together with three previously reported sesquiterpenoids (7-9). In addition three new oplopananes (2a-4a) were also obtained by chemical transformation. All structures of these sesquiterpenoids were established based on the comprehensive spectroscopic analyses, including NMR, MS, and IR, and comparing with the literatures.
[Sesquiterpenoids from rhizome of Homalomena occulta].[Pubmed: 28905602]
Twelve compounds were isolated from alcohol extracts of the rhizome of Homalomena occulta by using various chromatographic techniques including column chromatography onsilica gel and C₁₈ reverse-phase silica gel, and semi-preparative HPLC. Their structures were identified by physico-chemical properties and spectroscopic data analysis as 3α, 7α-dihydroxy-cadin-4-ene (1), 3-oxofabiaimbricatan (2), 3β, 4α-dihydroxy-7-epi-eudesm-11(13)-ene (3), integrifonol A(4), 1β, 6β-dihydroxy-7-epi-eudesm-11(13)-ene (5), 4β, 7β, 11-enantioeudesmantriol (6), epi-guaidiol (7), oplopanone(8), (-)-1β, 4β, 6α-trihydroxy-eudesmane (9),2α-hydroxyhomalomenol(10), (-)-T-muurolol (11) and hamalomenol A(12). Compounds 1-7 were obtained from the genus Homalomena for the first time and 11-12 were firstly reported from the species. Additionally, compounds 3, 5 and 8 displayed inhibitory effects against the lipopolysaccharide (LPS)-induced nitric oxide (NO) production in mouse macrophage RAW264.7 cells with IC₅₀ values of 6.51, 3.25, 7.78 μmol•L⁻¹, respectively.
New sesquiterpenes from the rhizomes of homalomena occulta.[Pubmed: 26718734]
Six new sesquiterpenes (1-6), along with eight known ones (7-14) were isolated from the rhizomes of Homalomena occulta. Structure elucidation of the new compounds was achieved through 1D NMR, 2D NMR spectroscopic techniques and HRESIMS, while the absolute configurations of compounds 1, 2 and 5 were confirmed by X-ray crystallographic analysis. All of the isolates were evaluated for their activity against LPS-induced production of nitrogen oxide (NO) in macrophage cells, and compounds 1 and 5 showed inhibitory effect on NO production with the IC50 values of 21.2 and 15.4 μM, respectively.
Preparation of molecularly imprinted polymers based on magnetic nanoparticles for the selective extraction of protocatechuic acid from plant extracts.[Pubmed: 25641806]
In this study, highly selective core-shell molecularly imprinted polymers on the surface of magnetic nanoparticles were prepared using protocatechuic acid as the template molecule. The resulting magnetic molecularly imprinted polymers were characterized by transmission electron microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and vibrating sample magnetometry. The binding performances of the prepared materials were evaluated by static and selective adsorption. The binding isotherms were obtained for protocatechuic acid and fitted by the Langmuir isotherm model and Freundlich isotherm model. Furthermore, the resulting materials were used as the solid-phase extraction materials coupled to high-performance liquid chromatography for the selective extraction and detection of protocatechuic acid from the extracts of Homalomena occulta and Cynomorium songaricum with the recoveries in the range 86.3-102.2%.
A new sesquiterpenoid from the rhizomes of Homalomena occulta.[Pubmed: 25104218]
Chemical constituents of EtOAc extract from the rhizomes of traditional Chinese medicine Qian-nian-jian (Homalomena occulta) have been studied, a new sesquiterpenoid, named euadesma-4-ene-1β,15-diol (1), and four related known compounds, polydactin B (2), oplodiol (3), 1β,4β,7α-trihydroxyeudesmane (4), and (-)1β,4β,6α-trihydroxy-eudesmane (5), were isolated. Their structures were elucidated using spectroscopic methods including 1D and 2D NMR techniques and mass spectrometry. All the isolates were tested against the human lung adenocarcinoma A549 using MTT assay method. Oplodiol (3) and (-)1β,4β,6α-trihydroxy-eudesmane (5) were found to show moderate cytotoxic effects on A549 with IC50 values at 25.5 and 15.0 μg/mL, respectively.
[Chemical constituents from rizomes of Homalomena occulta].[Pubmed: 24199565]
Column chromatography on silica gel and Sephadex LH-20 was used to study the chemical constituents of Homalomena occulta. The chemical structures of the separated compounds were elucidated by spectroscopic data analyseS. Twelve compounds were obtained and identified as 5-pentylresorcinol-b-glucoside (1), protocatechuic acid (2), 4-hydroxybenzoic acid (3), vanillic acid (4), 5-hydroxymethyl-2-furancarboxylic acid (5), 2-furoic acid (6), 5-hydroxymethyl-2-furfural (7), (R) -malic acid (8), (R) -dimethyl malate (9), trimethyl 1,2,3-propanetricarboxylate (10), 4-hydroxytetrahydrofuran-2-one (11) and (1S, 2S, 4S)-p-menthane-1,2, 4-triol (12). Among them, compound 1 was a new natural product, and compounds 4-12 were isolated from the genus for the first time.
Sesquiterpenoids from the rhizomes of Homalomena occulta.[Pubmed: 22573367]
Phytochemical investigation on the rhizomes of Homalomena occulta resulted in the isolation of five new sesquiterpenoids, namely cadinane-4β,5α,10α-triol (1), 5(11)-epoxycadinane-4β,5β,10β,11-tetraol (2), bullatantiol-1β-methyl malate (3), 1β,4β,7α-trihydroxyeudesmane-1β-methyl malate (6), and 1β,4α,7-trihydroxyeudes-mane (7), together with five known sesquiterpenoids, bullatantriol (4), acetylbullatantriol (5), 1β,4β,7α-trihydroxyeudesmane (8), 1β,4β,7β-trihydroxyeude-smane (9), and pterodontriol (10). Their structures were elucidated on the basis of spectroscopic evidences, including various 1D and 2D NMR and HR-ESI-MS. The structure of 1 was further confirmed by single-crystal X-ray diffraction analysis.
BACE1 (beta-secretase) inhibitory phenolic acids and a novel sesquiterpenoid from Homalomena occulta.[Pubmed: 20397233]
Four phenolic acids, namely 2-[(Z)-heptadec-11-enyl]-6-hydroxybenzoic acid (1), 2-[(6Z,9Z,12Z)-heptadeca-6,9,12-trienyl]-6-hydroxybenzoic acid (2), 2-[(9Z,12Z)-heptadeca-9,12-dienyl]-6-hydroxybenzoic acid (3), and 2-hydroxy-6-(12-phenyldodecyl)benzoic acid (4), and one sesquiterpene, asperpenoid (5), were isolated from the 95% EtOH extract of the roots of Homalomena occulta, among which 1, 2, and 5 represent new compounds. Further, the phenolic acids 1-4 exhibited BACE1 (beta-secretase) inhibitory activity with IC(50) values of 6.23+/-0.94, 6.28+/-0.63, 7.93+/-0.38, and 7.65+/-0.62 microM, respectively.
A new sesquiterpenoid from rhizomes of Homalomena occulta.[Pubmed: 19735040]
A new sesquiterpeniod, 6alpha, 7alpha, 10alpha-trihydroxyisoducane (1), together with two known sesquiterpenoids, oplodiol (2) and oplopanone (3), were isolated from the chloroform extract of the rhizomes of Homalomena occulta. Their structures were elucidated by 1D and 2D NMR spectral interpretation.
Sesquiterpenoids from Homalomena occulta affect osteoblast proliferation, differentiation and mineralization in vitro.[Pubmed: 18649899]
Chemical investigation of rhizomes of Homalomena occulta (Lours) resulted in isolation and identification of two sesquiterpenoids (6,7), and one daucane ester 8, together with five known sesquiterpenoids, oplodiol, oplopanone, homalomenol C, bullatantriol, and 1beta,4beta,7alpha-trihydroxyeudesmane. Their structures were elucidated using 1D and 2D NMR spectroscopic and X-ray analyses. The chloroform extract of this plant and compounds 1-7 were tested in vitro for their activities in stimulating osteoblast (OB) proliferation, differentiation and mineralization. Compounds 1-4 had a stimulative effect on significantly proliferation and differentiation of culture osteoblasts, while the chloroform extract and 1 significantly stimulated mineralization of cultured osteoblasts in vitro.


