Illicium simonsii
Illicium simonsii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Illicium simonsii
- Cat.No. Product Name CAS Number COA
-
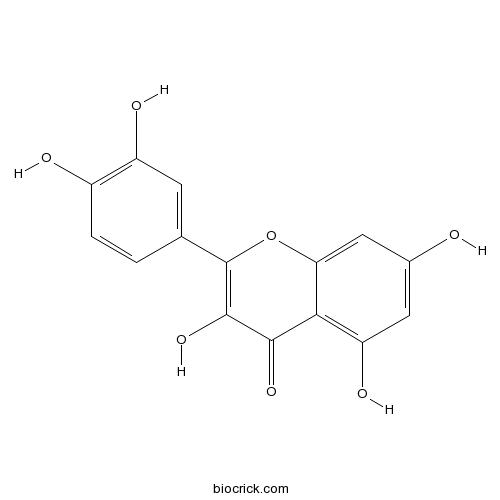
BCN6049
Quercetin117-39-5
Instructions
-
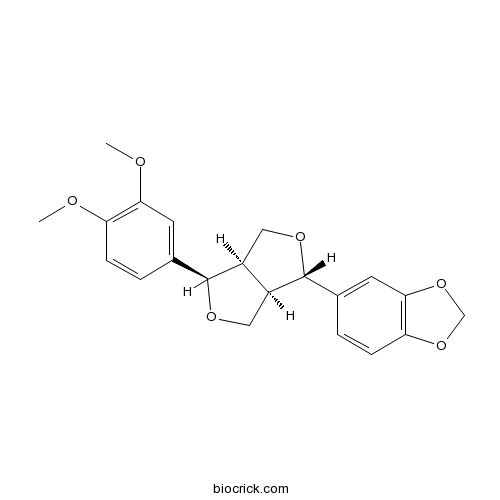
BCN5022
Fargesin31008-19-2
Instructions
-
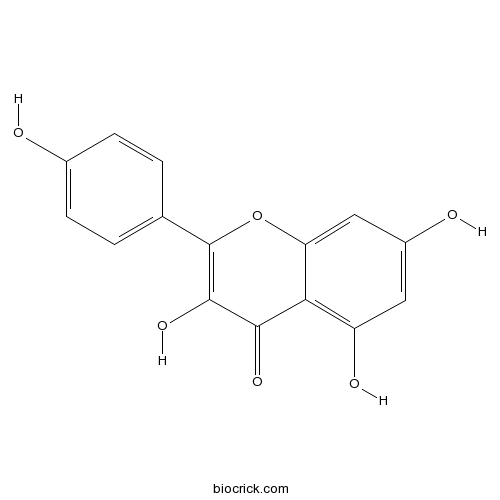
BCN5653
Kaempferol520-18-3
Instructions
Hot off the press.[Pubmed: 29350723]
A personal selection of 32 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as illisimonin A from Illicium simonsii.
Neolignan and phenylpropanoid compounds from the fruits of Illicium simonsii Maxim.[Pubmed: 29298500]
None
Simonols A and B, two novel sesqui-neolignans from the fruits of Illicium simonsii.[Pubmed: 23583436]
Two new sesqui-neolignans with novel conjugation way, simonol A (1), featuring a unique motif of a 5,5-dihydro-pyran with a hemiketal carbon, while simonol B (2) possessing two dihydronfuran rings in the same direction, were isolated from the ethanol extract of the fruits of Illicium simonii. Their structures were elucidated by spectroscopic methods including 1D and 2D NMR, HRESIMS, and calculation of electronic circular dichroism (ECD) using density functional theory (DFT). The two isolates were evaluated for their inhibitory activities against the growth of four lines of human cancer cells (NCI-H460, SMMC-7721, MCF-7, BGC-823): 1 showed strong activities comparable to 5-Fluorouracil, and 2 to a less content. In addition, plausible biosynthetic routes for the two compounds were also proposed.
Sesquineolignans and terpene-sesquineolignans: anti-acetylcholinesterase constituents from Illicium simonsii.[Pubmed: 23468312]
Chemical investigation of the aerial parts of Illicium simonsii resulted in the isolation of nine new compounds, simonsols A-E (1-5), simonsins A-B (7-8), terpene-sesquineolignans, clovanedunnianol (9), and p-menthadunnianol (10). Compound 5 was equilibrated with 7 as an inseparable mixture. The structures were elucidated by extensive NMR and MS analysis. Compounds 9, 10, and the mixture of 5 and 7 moderately inhibited acetylcholinesterase with IC50 values of 4.58 µM, 6.55 µM, and 10.34 µM, respectively.
A new phenylpropanoid glycoside from the fruits of Illicium simonsii.[Pubmed: 23302524]
To study the constituents and bioactivity of Illicium simonsii.
Reversal effects of components from the fruits of Illicium simonsii on human Adriamycin-resistant MCF-7 and 5-fluorouracil-resistant Bel7402 cells.[Pubmed: 21953821]
Twenty-one compounds including seven characteristic sesquiterpene lactones were isolated from Illicium simonsii and screened in vitro for their potential to restore the sensitivity of Adriamycin (ADR) resistant breast cancer cells (MCF-7/ADR) and 5-fluorouracil-resistant hepatocellular carcinoma cells (Bel7402/5-FU) to ADR and 5-fluorouracil, respectively. These compounds were found to be non-toxic to a panel of tumour cell lines: human breast cancer cells (MCF-7), cervical cancer cells (HeLa), hepatic liver carcinoma cells (HepG-2, Bel7402) and gastric carcinoma cells (BGC-823, SGC-7901). Five compounds showed an obvious decrease in the IC(50) of doxorubicin in MCF-7/ADR and four compounds sensitized Bel7402/5-FU to 5-fluorouracil at non-toxic concentrations. The relationship between the structure of these non-cytotoxic substances and their multidrug-resistant (MDR) reversal abilities was investigated by principal component analysis (PCA) of the reversing fold (RF) values of these compounds and their calculated molecular descriptors of the tested substances. No correlations were found between the reversal potencies of these compounds to the two MDR cells. Compounds with lower polarity generally had stronger sensitizing ability to the P-glycoprotein (Pgp) overexpressed MCF-7/ADR cells. On the other hand, higher hydrophilic compounds seemed to exhibit a stronger reversal effect to the MDR-associated protein (MRP) overexpressed Bel7402/5-FU cell line. Our findings favoured further investigations on the active substances and the underlying mechanisms.
[Studies on chemical constituents of Illicium simonsii].[Pubmed: 21837972]
To study the chemical constituents of the Illicium simonsii.
Two new phenylpropanoid derivatives and other constituents from Illicium simonsii active against oral microbial organisms.[Pubmed: 20195962]
Bioassay-guided fractionation of the ethanol extract of the leaves and stems of Illicium simonsii led to the isolation of two new compounds, simonin A (1) and 1-hydroxyl-2- O- β- D-6'-acetyl-glucopyranosyl-4-allylbenzene (2), along with eight known compounds. Their structures were elucidated by spectroscopic analysis. The isolates were tested for anti-oral microbial activity using a microdilution method. Compounds 1 and 3- 6 showed significant activities against oral microbial organisms (Actinomyces viscosus, Streptococcus mutans, Streptococcus sanguis, and Actinomyces naeslundii), with minimum inhibitory concentration (MIC) values ranging from 1.95 to 31.25 µg/mL in vitro.
Prenylated C6-C3 compounds from the fruits of Illicium simonsii.[Pubmed: 20183276]
Two new prenylated C(6)-C(3) compounds, 4-epi-illicinone E-12-shikimate (1) and 3-hydroxyillifunone B (2), together with five known prenylated C(6)-C(3) compounds (3-7), were isolated from the fruits of Illicium simonsii. Their structures were elucidated on the basis of extensive spectroscopic methods, including 1D and 2D NMR, CD spectra, and ESI-MS analysis.


