Iris germanica
Iris germanica
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Iris germanica
- Cat.No. Product Name CAS Number COA
-
BCN3849
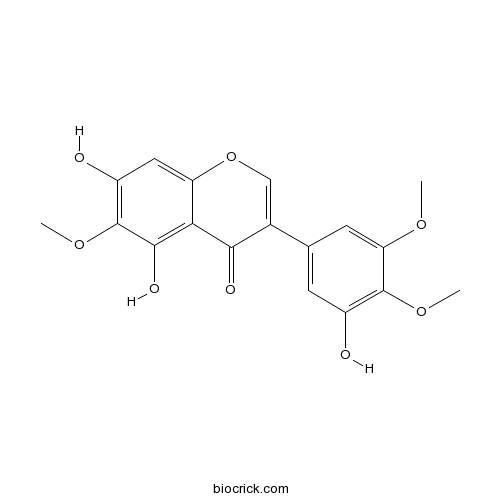
Irigenin548-76-5
Instructions
8-Hydroxyirilone 5-methyl ether and 8-hydroxyirilone, new antioxidant and α-amylase inhibitors isoflavonoids from Iris germanica rhizomes.[Pubmed: 28069265]
None
Aggregation of Thaumatomyia glabra (Diptera: Chloropidae) Males on Iris spp. Flowers Releasing Methyl Anthranilate.[Pubmed: 28028094]
Aggregations of Thaumatomyia glabra (Diptera: Chloropidae) were observed on flowers of Iris pallida Lamarck (Asparagales: Iridaceae), whereas no T. glabra (Meigen) were observed on nearby Iris germanica L. flowers. Sampling of T. glabra on I. pallida flowers revealed the presence of males only. In a previous study, T. glabra males were attracted to methyl anthranilate. We found methyl anthranilate in extracts of I. pallida flowers on which T. glabra aggregated, but not in extracts of I. germanica flowers. Applying methyl anthranilate to I. germanica flowers elicited attraction of T. glabra to the flowers. This study suggests that I. pallida flowers may attract T. glabra males to aggregate because they release the known attractant, methyl anthranilate, whereas I. germanica flowers may not be attractive because they do not release methyl anthranilate.
Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina.[Pubmed: 26166432]
This study was designed to investigate antioxidant and anticholinesterase potential of Iris germanica var; florentina. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory potential of plant samples were investigated by Ellman's assay. Antioxidant activity was performed using DPPH, H2O2 and ABTS free radical scavenging assays. Total phenolics and flavonoids contents were expressed in mg GAE/g dry weight and mg RTE/g, respectively. In AChE inhibition assay, Ig.Fl, Ig.Sp and Ig.Cf fractions exhibited highest activity with IC50 values of < 0.1, 5.64 and 19 μg/mL, respectively. In BChE inhibitory assay, Ig.Fl, Ig.Sp, Ig.Cf and Ig.Cr were most active with IC50 of < 0.1, < 0.1, 31 and 78 μg/mL, respectively. In DPPH assay, Ig.Fl and Ig.Cf exhibited highest inhibition of free radicals, 80.52% (IC50 = 9 μg/mL) and 78.30% (IC50 = 8 μg/mL), respectively. In ABTS assay Ig.Cr, Ig.Cf, Ig.Fl and Ig.Sp exhibited IC50 values of < 0.1, 2, 2 and 3 μg/mL, respectively.
A metabolomic approach to quality determination and authentication of raw plant material in the fragrance field. Iris rhizomes: a case study.[Pubmed: 25441349]
This study aimed to discriminate 22 samples of commercial Iris rhizomes (orris root) by species and origin (Iris germanica (Morocco), I. albicans (Morocco), I. pallida (Morocco), I. pallida (China), I. pallida (Italy)) by applying a strategy derived from those adopted in metabolomics. The specimens' fingerprints from conventional analysis methods (LC-UV and/or LC-MS) were unable to provide clear discrimination. A strategy combining UHPLC/TOF-HRMS, in positive and negative modes, with multivariate statistical methods was therefore applied. Exact mass/retention time (EMRT) pairs obtained by UHPLC-TOF/HRMS were successfully submitted to statistical processing by principal component analysis (PCA), partial least square discriminant analysis (PLS-DA), and then orthogonal partial least square-discriminant analysis (OPLS-DA), to extract the discriminating EMRT pairs through their trend views. 146 EMRT pairs were selected on the basis of their trend views, because they significantly varied, and 104 of them were included to discriminate between species and origins. 32 of them were tentatively identified as discriminating markers (flavonoids, isoflavonoids, triterpenoids, benzophenone derivatives and related glycosides …) from the reference database created on the basis of Iris genus components reported in the literature: eight of them specific for I. albicans, four for I. germanica, five for I. pallida (Italy), five for I. pallida (China), and ten for I. pallida (Morocco). The reliability of this strategy was confirmed by identifying species and origin of two unknown samples submitted to the same analytical procedure.
[Chemical constituents from rhizomes of Iris germanica].[Pubmed: 25204177]
Twenty-one compounds were isolated from the rhizomes of Iris germanica by various chromatographic techniques such as silica gel, ODS and Sephadex LH-20 chromatography. Their structures were established on basis of physical properties, MS and NMR spectroscopic data Their structures were identified as ombuin (1), 5, 3, 3'-trihydroxy-7, 4'-dimethoxyflavanone (2), naringenin (3), cirsiliol-4'-glucoside (4), 3beta, 4'-dihydroxy-7,3'-dimethoxyflavonone-5-O-beta-D-glucopyranoside (5), genistein (6), irilin D (7), muningin (8), 5, 7, 4'-trihydroxy-6, 3', 5'-trimethoxyisoflavone (9), tectorigenin (10), irigenin (11), tectoridin (12), iridin (13), mangiferin (14), irisxanthone (15), pyroglutamic acid (16), 2, 4', 6-trihydroxy-4-methoxybenzophenone-2-O-beta-D-glucoside (17), apocynin (18), androsin (19), beta-sitosterol (20), and daucosterol (21). Among them, compounds 1-9, 16, 17 were obtained from this plant for the first time, compounds 8 and 9 were separated from Iris species for the first time, compounds 1, 4, and 17 were obtained from the family for the first time.
Alteration of flower color in Iris germanica L. 'Fire Bride' through ectopic expression of phytoene synthase gene (crtB) from Pantoea agglomerans.[Pubmed: 24801678]
Genetic modulation of the carotenogenesis in I. germanica 'Fire Bride' by ectopic expression of a crtB gene causes several flower parts to develop novel orange and pink colors. Flower color in tall bearded irises (Iris germanica L.) is determined by two distinct biochemical pathways; the carotenoid pathway, which imparts yellow, orange and pink hues and the anthocyanin pathway, which produces blue, violet and maroon flowers. Red-flowered I. germanica do not exist in nature and conventional breeding methods have thus far failed to produce them. With a goal of developing iris cultivars with red flowers, we transformed a pink iris I. germanica, 'Fire Bride', with a bacterial phytoene synthase gene (crtB) from Pantoea agglomerans under the control of the promoter region of a gene for capsanthin-capsorubin synthase from Lilium lancifolium (Llccs). This approach aimed to increase the flux of metabolites into the carotenoid biosynthetic pathway and lead to elevated levels of lycopene and darker pink or red flowers. Iris callus tissue ectopically expressing the crtB gene exhibited a color change from yellow to pink-orange and red, due to accumulation of lycopene. Transgenic iris plants, regenerated from the crtB-transgenic calli, showed prominent color changes in the ovaries (green to orange), flower stalk (green to orange), and anthers (white to pink), while the standards and falls showed no significant differences in color when compared to control plants. HPLC and UHPLC analysis confirmed that the color changes were primarily due to the accumulation of lycopene. In this study, we showed that ectopic expression of a crtB can be used to successfully alter the color of certain flower parts in I. germanica 'Fire Bride' and produce new flower traits.
New isoflavones with cytotoxic activity from the rhizomes of Iris germanica L.[Pubmed: 23662687]
Two new compounds, 5-methoxy-3',4'-dihydroxy-6,7-methylenedioxy-4H-1-benzo-pyran-4-one(iriskashmirianin A) (1) and 5,3'-dihydroxy-3-(4'-β-D-glucopyranosyl)-6,7-methylenedioxy-4H-1-benzo-pyran-4-one (germanaism H) (2), along with eight known compounds (3-10), were isolated from the rhizomes of Iris germanica L. The cytotoxicities of these compounds were tested using Ehrlich's ascites carcinoma (EAC) cancer cell line by 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazoli-umbromide (MTT) and ATP assays. The results showed that these compounds possessed antiproliferative effects on EAC cell line. Among them, compound 1 possessed the best cytotoxic activity with IC50 ± SD of 20.9 ± 2.7 and 4.3 ± 0.9 μM for MTT and ATP assay methods, respectively.
Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium Thunb. 'Splendens').[Pubmed: 23008421]
The orange color of tiger lily (Lolium lancifolium 'Splendens') flowers is due, primarily, to the accumulation of two κ-xanthophylls, capsanthin and capsorubin. An enzyme, known as capsanthin-capsorubin synthase (CCS), catalyzes the conversion of antheraxanthin and violaxanthin into capsanthin and capsorubin, respectively. We cloned the gene for capsanthin-capsorubin synthase (Llccs) from flower tepals of L. lancifolium by the rapid amplification of cDNA ends (RACE) with a heterologous non-degenerate primer that was based on the sequence of a gene for lycopene β-cyclase (lcyB). The full-length cDNA of Llccs was 1,785 bp long and contained an open reading frame of 1,425 bp that encoded a polypeptide of 474 amino acids with a predicted N-terminal plastid-targeting sequence. Analysis by reverse transcription-PCR (RT-PCR) revealed that expression of Llccs was spatially and temporally regulated, with expression in flower buds and flowers of L. lancifolium but not in vegetative tissues. Stable overexpression of the Llccs gene in callus tissue of Iris germanica, which accumulates several xanthophylls including violaxanthin, the precursor of capsorubin, resulted in transgenic callus whose color had changed from its normal yellow to red-orange. This novel red-orange coloration was due to the accumulation of two non-native κ-xanthophylls, capsanthin and capsorubin, as confirmed by HPLC and ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis with authentic standards. Cloning of the Llccs gene should advance our understanding of the molecular and genetic mechanisms of the biosynthesis of κ-carotenoids in general and in the genus Lilium in particular, and will facilitate transgenic alterations of the colors of flowers and fruits of many plant species.


