IrigeninCAS# 548-76-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 548-76-5 | SDF | Download SDF |
| PubChem ID | 5464170 | Appearance | White powder |
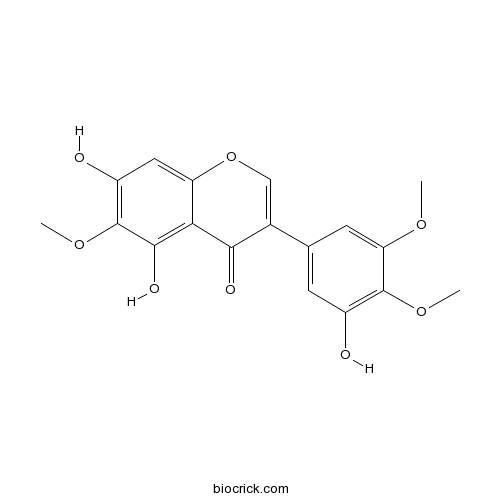
| Formula | C18H16O8 | M.Wt | 360.31 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 3',5,7-Trihydroxy 4',5',6-trimethoxyisoflavone | ||
| Solubility | Soluble in ethanol and methan | ||
| Chemical Name | 5,7-dihydroxy-3-(3-hydroxy-4,5-dimethoxyphenyl)-6-methoxychromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1OC)O)C2=COC3=CC(=C(C(=C3C2=O)O)OC)O | ||
| Standard InChIKey | TUGWPJJTQNLKCL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O8/c1-23-13-5-8(4-10(19)17(13)24-2)9-7-26-12-6-11(20)18(25-3)16(22)14(12)15(9)21/h4-7,19-20,22H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Irigenin, an α-glucosidase inhibitor, which has anti-inflammatory, anti-cancer, and anti-metastatic effects. Irigenin can inhibit the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 proteins and mRNAs without an appreciable cytotoxic effect. Irigenin displays moderate activity as inducers of NAD(P)H:quinone reductase (QR) in cultured mouse Hepa 1c1c7 cells. |
| Targets | COX | NOS | NAD(P)H:quinone reductase | α-glucosidase | NO | PGE | p65 | NF-κB |
| In vivo | Irigenin, a novel lead from Western Himalayan chemiome inhibits Fibronectin-Extra Domain A induced metastasis in Lung cancer cells.[Pubmed: 27849000 ]Sci Rep. 2016 Nov 16;6:37151.Several lines of evidence indicate that Fibronectin Extra Domain A (EDA) promotes metastatic capacity of tumor cells by engaging cell surface α9β1 integrins. This interaction mediated by the C-C loop of EDA activates pro-oncogenic signaling pathways leading to epithelial to mesenchymal transition (EMT) of tumor cells, thus signifying its importance in control of metastatic progression. |
| Kinase Assay | Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells.[Pubmed: 16307761 ]Life Sci. 2006 Apr 11;78(20):2336-42.
|
| Cell Research | Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica.[Pubmed: 12567273 ]Planta Med. 2003 Jan;69(1):15-20.
|
| Structure Identification | J Sep Sci. 2017 Jun;40(12):2565-2574.Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential α-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography.[Pubmed: 28444982 ]The separation of a compound of interest from its structurally similar homologues to produce high-purity natural products is a challenging problem. |

Irigenin Dilution Calculator

Irigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7754 mL | 13.8769 mL | 27.7539 mL | 55.5078 mL | 69.3847 mL |
| 5 mM | 0.5551 mL | 2.7754 mL | 5.5508 mL | 11.1016 mL | 13.8769 mL |
| 10 mM | 0.2775 mL | 1.3877 mL | 2.7754 mL | 5.5508 mL | 6.9385 mL |
| 50 mM | 0.0555 mL | 0.2775 mL | 0.5551 mL | 1.1102 mL | 1.3877 mL |
| 100 mM | 0.0278 mL | 0.1388 mL | 0.2775 mL | 0.5551 mL | 0.6938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetagetin-7-O-glucoside
Catalog No.:BCN6480
CAS No.:548-75-4
- Crystal Violet
Catalog No.:BCC4772
CAS No.:548-62-9
- Cornin
Catalog No.:BCN5008
CAS No.:548-37-8
- Oxysanguinarine
Catalog No.:BCN8100
CAS No.:548-30-1
- Isolariciresinol
Catalog No.:BCN5727
CAS No.:548-29-8
- Isoginkgetin
Catalog No.:BCN2320
CAS No.:548-19-6
- Roemerine
Catalog No.:BCN8236
CAS No.:548-08-3
- Hypericin
Catalog No.:BCN5977
CAS No.:548-04-9
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- JTE 013
Catalog No.:BCC7348
CAS No.:547756-93-4
- Fluvoxamine
Catalog No.:BCC4214
CAS No.:54739-18-3
- 4-(4-Hydroxyphenyl)-2-butanone
Catalog No.:BCN6797
CAS No.:5471-51-2
- Tectorigenin
Catalog No.:BCN1019
CAS No.:548-77-6
- Pinobanksin
Catalog No.:BCN5729
CAS No.:548-82-3
- Galangin
Catalog No.:BCN5730
CAS No.:548-83-4
- Gyrophoric acid
Catalog No.:BCN5731
CAS No.:548-89-0
- Trichodesmine
Catalog No.:BCN2145
CAS No.:548-90-3
- Protoveratrine B
Catalog No.:BCN2435
CAS No.:124-97-0
- Daphneolone
Catalog No.:BCN3230
CAS No.:54835-64-2
- Roseoside
Catalog No.:BCN5728
CAS No.:54835-70-0
- Protogracillin(P)
Catalog No.:BCC8352
CAS No.:54848-30-5
- Sanshodiol
Catalog No.:BCN6577
CAS No.:54854-91-0
- PD 334581
Catalog No.:BCC6300
CAS No.:548756-68-9
- Arborinine
Catalog No.:BCN7438
CAS No.:5489-57-6
Cancer chemopreventive in vitro activities of isoflavones isolated from Iris germanica.[Pubmed:12567273]
Planta Med. 2003 Jan;69(1):15-20.
Six known isoflavones were isolated from the rhizomes of Iris germanica, and were established by UV, MS and NMR techniques as irisolidone (1), irisolidone 7-O-alpha-D-glucoside (1a), Irigenin (2), irilone (3), iriflogenin (4), and iriskashmirianin (5). These compounds were examined for their cancer chemopreventive potential. They were shown to be potent inhibitors of cytochrome P450 1A activity with IC 50 values in the range 0.25-4.9 microM. The isoflavones 2, 3 and 5 displayed moderate activity as inducers of NAD(P)H:quinone reductase (QR) in cultured mouse Hepa 1c1c7 cells, with CD values (concentration required to double the specific activity of QR) of 3.5-16.7 microM, whereas weak activity was observed with compounds 4 and 5 in the radical (DPPH) scavenging bioassay (IC 50 values 89.6 and 120.3 microM, respectively). With respect to anti-tumor promoting potential based on anti-inflammatory mechanisms, none of the compounds demonstrated significant activity in the concentration range tested.
Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells.[Pubmed:16307761]
Life Sci. 2006 Apr 11;78(20):2336-42.
In the present study, we investigated antiinflammatory effects of six flavonoids isolated from the rhizomes of Belamcanda chinensis (Iridaceae) in RAW 264.7 macrophages. The results indicated that Irigenin concentration dependently inhibited lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin (PG) E(2) production. Furthermore, this compound inhibited the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 proteins and mRNAs without an appreciable cytotoxic effect. Treatment of the transfectant RAW 264.7 cells with Irigenin reduced the level of nuclear factor-kappaB (NF-kappaB) activity, also effectively lowered NF-kappaB binding measured by electrophoretic mobility shift assay (EMSA), which was associated with decreased p65 protein levels in the nucleus. On the basis of the above data, we suggest that the effect of Irigenin in decreasing LPS-induced NO and PGE(2) synthesis is due to diminish the mRNA and protein expression of iNOS and COX-2, respectively, also may be due to under the suppression of NF-kappaB activation. Therefore, Irigenin isolated from the rhizomes of Belamcanda chinensis could be offered as a leading compound for anti-inflammation.
Ionic-liquid-based ultrasound-assisted extraction of isoflavones from Belamcanda chinensis and subsequent screening and isolation of potential alpha-glucosidase inhibitors by ultrafiltration and semipreparative high-performance liquid chromatography.[Pubmed:28444982]
J Sep Sci. 2017 Jun;40(12):2565-2574.
The separation of a compound of interest from its structurally similar homologues to produce high-purity natural products is a challenging problem. This work proposes a novel method for the separation of iristectorigenin A from its structurally similar homologues by ionic-liquid-based ultrasound-assisted extraction and the subsequent screening and isolation of potential alpha-glucosidase inhibitors via ultrafiltration and semipreparative high-performance liquid chromatography. Ionic-liquid-based ultrasound-assisted extraction was successfully applied to the extraction of tectorigenin, iristectorigenin A, Irigenin, and irisflorentin from Belamcanda chinensis. The optimum conditions for the efficient extraction of isoflavones were determined as 1.0 M 1-ethyl-3-methylimidazolium tetrafluoroborate with extraction time of 30 min and a solvent to solid ratio of 30 mL/g. Ultrafiltration with liquid chromatography and mass spectrometry was applied to screen and identify alpha-glucosidase inhibitors from B. chinensis, followed by the application of semipreparative high-performance liquid chromatography to separate and isolate the active constituents. Four major compounds including tectorigenin, iristectorigenin A, Irigenin, and irisflorentin were screened and identified as alpha-glucosidase inhibitors, and then the four active compounds abovementioned were subsequently isolated by semipreparative high-performance liquid chromatography (99.89, 88.97, 99.79, and 99.97% purity, respectively). The results demonstrate that ionic liquid extraction can be successfully applied to the extraction of isoflavones from B. chinensis.
Irigenin, a novel lead from Western Himalayan chemiome inhibits Fibronectin-Extra Domain A induced metastasis in Lung cancer cells.[Pubmed:27849000]
Sci Rep. 2016 Nov 16;6:37151.
Several lines of evidence indicate that Fibronectin Extra Domain A (EDA) promotes metastatic capacity of tumor cells by engaging cell surface alpha9beta1 integrins. This interaction mediated by the C-C loop of EDA activates pro-oncogenic signaling pathways leading to epithelial to mesenchymal transition (EMT) of tumor cells, thus signifying its importance in control of metastatic progression. In this context the present study was designed to explore the active compounds from selected ethno-medicinal plants of western Himalayan region for targeting EDA of Fibronectin in lung carcinoma cells. Structure based informatics for drug designing and screening was employed to generate a lead compound(s) feed that were conformationally and energetically viable. Out of 120 compounds selected, Irigenin showed best binding-affinity with C-C loop of EDA. Irigenin specifically targeted alpha9beta1 and alpha4beta1 integrin binding sites on EDA comprising LEU46, PHE47, PRO48, GLU58, LEU59 and GLN60 in its C-C loop as evaluated by energy decomposition per residue of Irigenin-EDA complex. In-vitro cell motility assays complemented with EDA knock-in and knockdown assays distinctively demonstrated that Irigenin prevents metastatic capacity of lung cancer cells by selectively blocking EDA. The results presented thus project Irigenin as a lead compound to overcome Fibronectin EDA induced metastatic progression in lung carcinoma cells.


