Iris tectorum
Iris tectorum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Iris tectorum
- Cat.No. Product Name CAS Number COA
-
BCN3849
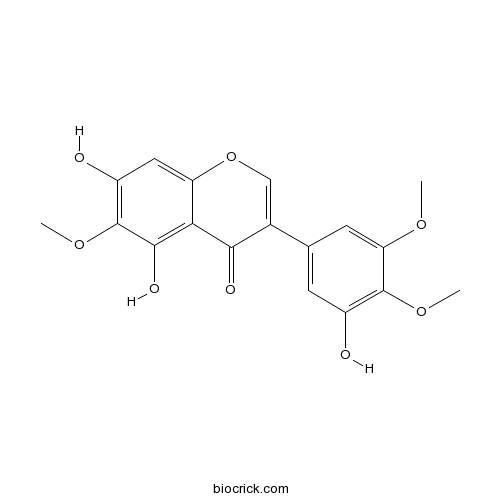
Irigenin548-76-5
Instructions
-
BCN1020
Tectoridin611-40-5
Instructions

-
BCN2762
Swertisin6991-10-2
Instructions

[Determination of six active ingredients in different parts of Belamcanda chinensis and Iris tectorum and their anti-inflammatory activity].[Pubmed: 29552821]
In order to explore the anti-inflammatory activity and active ingredient basis from the leaves of the Belamcanda chinensis and Iris tectorum, we established an HPLC method for simultaneous determination of six anti-inflammatory active ingredient contents in the root of the B. chinensis and I. tectorum as well as their leaves with different dry methods, and the anti-inflammatory effects of the extract were studied by the mouse ear swelling experiment. The HPLC analysis was performed on an Agilent WondaSil© C₁₈-WR(4.6 mm×250 mm,5 μm),with isocratic elution of acetonitrile-0.1% ortho-phosphoric acid solution at a flow rate of 1. 0 mL·min⁻¹ and the detection was carried out at 265 nm. The chemical compositions of the B. chinensis and I. tectorum are similar but the contents of them are obviously different. Both rhizome and leaf extract of B. chinensis and I. tectorum had inhibitory effects on inflamed mice induced by dimethylbenzene and had anti-inflammatory effects by animal experiment, which could lay the material and active foundation for the development of the non-medicinal parts of the B. chinensis and I. tectorum.
Iritectol G, a novel iridal-type triterpenoid from Iris tectorum displays anti-epileptic activity in vitro through inhibition of sodium channels.[Pubmed: 28807716]
None
Apocynin derivatives from Iris tectorum.[Pubmed: 28081623]
Phytochemical investigation of the rhizomes of Iris tectorum resulted in the isolation and characterization of three new apocynin derivatives, apocynin-4-O-β-D-(6'-O-syringyl)glucopyranoside (1), scrophenoside C-7-ethyl ether (2, 3), together with a new naturally occurring compound but known by synthesis, apocynin-4-O-β-D-xylopyranoside (4), and five known ones (5-9). Their structures were elucidated on the basis of spectroscopic data interpretation.
Polycycloiridals with a Cyclopentane Ring from Iris tectorum.[Pubmed: 28032759]
None
Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species.[Pubmed: 27443461]
This study aims to investigate the removal of antibiotics and antibiotic resistance genes (ARGs) in raw domestic wastewater by various mesocosm-scale constructed wetlands (CWs) with different flow configurations or plant species including the constructed wetland with or without plant. Six mesocosm-scale CWs with three flow types (surface flow, horizontal subsurface flow and vertical subsurface flow) and two plant species (Thaliadealbata Fraser and Iris tectorum Maxim) were set up in the outdoor. 8 antibiotics including erythromycin-H2O (ETM-H2O), monensin (MON), clarithromycin (CTM), leucomycin (LCM), sulfamethoxazole (SMX), trimethoprim (TMP), sulfamethazine (SMZ) and sulfapyridine (SPD) and 12 genes including three sulfonamide resistance genes (sul1, sul2 and sul3), four tetracycline resistance genes (tetG, tetM, tetO and tetX), two macrolide resistance genes (ermB and ermC), two chloramphenicol resistance genes (cmlA and floR) and 16S rRNA (bacteria) were determined in different matrices (water, particle, substrate and plant phases) from the mesocosm-scale systems. The aqueous removal efficiencies of total antibiotics ranged from 75.8 to 98.6%, while those of total ARGs varied between 63.9 and 84.0% by the mesocosm-scale CWs. The presence of plants was beneficial to the removal of pollutants, and the subsurface flow CWs had higher pollutant removal than the surface flow CWs, especially for antibiotics. According to the mass balance analysis, the masses of all detected antibiotics during the operation period were 247,000, 4920-10,600, 0.05-0.41 and 3500-60,000μg in influent, substrate, plant and effluent of the mesocosm-scale CWs. In the CWs, biodegradation, substrate adsorption and plant uptake all played certain roles in reducing the loadings of nutrients, antibiotics and ARGs, but biodegradation was the most important process in the removal of these pollutants.
The inhibition and adaptability of four wetland plant species to high concentration of ammonia wastewater and nitrogen removal efficiency in constructed wetlands.[Pubmed: 26708488]
Four plant species, Typha orientalis, Scirpus validus, Canna indica and Iris tectorum were selected to assess their physiological response and effects on nitrogen and COD removal to high total ammoniacal nitrogen (TAN) in constructed wetlands. Results showed that high TAN caused decreased relative growth rate, net photosynthetic rate, and leaf transpiration. C. indica and T. orientalis showed higher TAN adaptability than S. validus and I. tectorum. Below TAN of 200 mg L(-1), growth of C. indica and T. orientalis was less affected or even stimulated at TAN range 100-200 mg L(-1). However, S. validus and I. tectorum was obviously suppressed when TAN was above 100 mg L(-1). High TAN generated obvious oxidative stress showing increased proline and malondialdehyde contents, and superoxide dismutase was inhibited. It indicated that the threshold for plant self-bioremediation against high TAN was 200 mg L(-1). What's more, planted CWs showed higher nitrogen and COD removal. Removal rate of C. indica and T. orientalis was higher than S. validus and I. tectorum.
Lignans from the rhizomes of Iris tectorum.[Pubmed: 26625840]
Chemical examination of the ethanol extract of rhizomes of Iris tectorum led to the isolation and characterization of three new lignans, (7R,7'R,8S,8'S)-5'-methoxy-neo-olivil (1a), (7S,7'S,8R,8'R) -5'-methoxy-neo-olivil (1b), (7S,7'R,8S,8'S)-neo-olivil (2a), (7R,7'S,8R,8'R)-neo-olivil (2b), (7R,7'R,8S,8'S,7''S,8''S)-threo-neo-olivil-4'-O-8-guaiacylglycerol ether (3), together with six known ones (4-9). Among them, compounds 1 and 2 were found to be racemic mixtures, respectively, which were verified by chiral HPLC analysis, compound 3 was a new sesquineolignan. The structures were elucidated on the basis of extensive spectroscopic analysis. To our knowledge, this is the first report of lignan constituents isolated from I. tectorum. All compounds were evaluated for their cytotoxicity against five human tumor cell lines and none of them displayed significant toxicity in tested cell lines at a concentration of 10 μM.
Polycycloiridals A-D, Four Iridal-Type Triterpenoids with an α-Terpineol Moiety from Iris tectorum.[Pubmed: 26555865]
Polycycloiridals A-D, four novel iridals with an unprecedented α-terpineol moiety resulting from cyclization of the homofarnesylside chain, were isolated from the ethanol extract of rhizomes of Iris tectorum. Their structures were elucidated on the basis of comprehensive spectroscopic analysis. The absolute configuration of 1 was determined by the modified Mosher's method and comparison of experimental and calculated electronic circular dichroism (ECD) spectrum. A possible biosynthetic pathway was postulated.
Comparative pharmacokinetic profiles of tectorigenin in rat plasma by UPLC-MS/MS after oral administration of Iris tectorum Maxim extract and pure tectoridin.[Pubmed: 26004225]
Iris tectorum Maxim, a well-known herb medicine, is commonly used for treatment of inflammation, cough, and pharyngitis for a long time in China. Tectoridin, main active ingredient of Iris tectorum Maxim, is often used for its quality control. This study was aimed to analyze the pharmacokinetic profile of tectorigenin (the metabolite of tectoridin) after oral administration of I. tectorum Maxim extract, and to compare the pharmacokinetic characterization of tectorigenin after oral administration of I. tectorum Maxim extract (ITME) and pure tectoridin (PT) in rats. In addition, a simple, reliable and sensitive UPLC-MS/MS method was developed for determination of tectorigenin in rat plasma, using kaempferol as internal standard. The processed samples were separated on a Poroshell 120 SB-C₁₈ column and detected by positive electrospray ionization in multiple reaction monitoring (MRM) mode. The method validation results indicated that the established method was simple, specific and reliable. The pharmacokinetic results showed that the plasma concentration of tectorigenin in ITME group was much higher than that of the PT group (p<0.01). Moreover, compared to PT group, t₁/₂ value and AUC(0-∞) value were also notably increased in ITME group (p<0.01). In conclusion, potential interaction exists between those chemical components in ITME, and the co-existing components in ITME could notably promote the absorption of tectoridin in rats, however, the exact compound(s) which enhance the absorption of tectoridin should be investigated in future study.
Fluorescence spectroscopy and docking study in two flavonoids, isolated tectoridin and its aglycone tectorigenin, interacting with human serum albumin: a comparison study.[Pubmed: 25920391]
Two flavonoids, tectoridin (TD) isolated from rhizomes of Iris tectorum and hydrolyzed aglycone tectorigenin (TG) were prepared and characterized to compare their different interaction ability with human serum albumin (HSA). Based on the results, the affinity of TG-HSA was stronger than that of TD-HAS, and TG combined more closely with HSA than did TD. HSA fluorescence was quenched by TD/TG. The interactions between TD/TG and HSA involved static quenching. The thermodynamic parameters indicated that both binding processes were spontaneous; hydrogen binding and van der Waals force were the main forces between TD and HSA, whereas a hydrophobic interaction was the main binding force between TG and HSA. Synchronous and 3D fluorescence spectra showed that the binding of TD/TG to HSA induced conformational changes. Moreover, a docking study confirmed the experimental results.


