Lepidium meyenii
Lepidium meyenii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Lepidium meyenii
- Cat.No. Product Name CAS Number COA
-
BCN9069
Benzyl alcohol100-51-6
Instructions

-
BCN1518
(9Z,12Z)-N-Benzyloctadeca-9,12-dienamide18286-71-0
Instructions

-
BCN8173
13-Oxo-9E,11E-octadecadienoic acid29623-29-8
Instructions

-
BCN8319
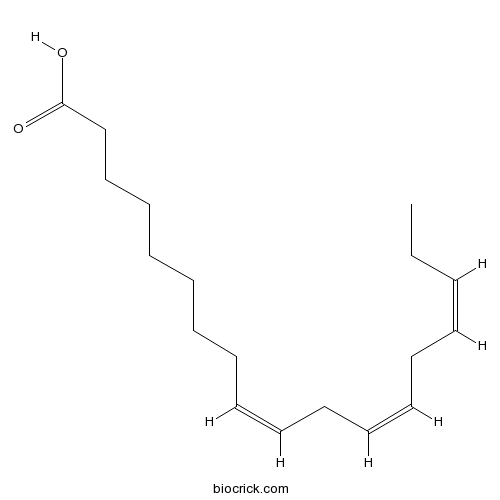
alpha-Linolenic acid463-40-1
Instructions
-
BCN3821
Linoleic acid60-33-3
Instructions

-
BCC3138
H-Val-OH72-18-4
Instructions

-
BCN1366
Macamide B74058-71-2
Instructions

-
BCN6531
N-Benzyllinolenamide883715-18-2
Instructions

-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Inhibition of Fatty Acid Amide Hydrolase (FAAH) by Macamides.[Pubmed: 29926378]
The pentane extract of the Peruvian plant, Lepidium meyenii (Maca), has been demonstrated to possess neuroprotective activity in previous in vitro and in vivo studies (Pino-Figueroa et al. in Ann N Y Acad Sci 1199:77-85, 2010; Pino-Figueroa et al. in Am J Neuroprot Neuroregener 3:87-92, 2011). This extract contains a number of macamides that may act on the endocannabinoid system (Pino-Figueroa et al. in Ann N Y Acad Sci 1199:77-85, 2010; Pino-Figueroa et al., 2011; Dini et al. in Food Chem 49:347-349, 1994). The aim of this study was to characterize the inhibitory activity of four of these maccamides (N-benzylstearamide, N-benzyloleamide, N-benzyloctadeca-9Z,12Z-dienamide, and N-benzyloctadeca-9Z,12Z,15Z-trienamide) on fatty acid amide hydrolase (FAAH), an enzyme that is responsible for endocannabinoid degradation in the nervous system (Kumar et al. in Anaesthesia 56:1059-1068, 2001). The four compounds were tested at concentrations between 1 and 100 μM, utilizing an FAAH inhibitor screening assay. The results demonstrated concentration-dependent FAAH inhibitory activities for the four macamides tested. N-Benzyloctadeca-9Z,12Z-dienamide demonstrated the highest FAAH inhibitory activity whereas N-benzylstearamide had the lowest inhibitory activity. In addition, N-benzylstearamide, N-benzyloleamide, and N-benzyloctadeca-9Z,12Z-dienamide demonstrated time-dependent inhibition when tested after a pre-incubation period, indicating that the mechanism of inhibition for these compounds most likely is irreversible. Of interest, unsaturation in the fatty acid moiety resulted in greater FAAH inhibitory activity. LC/MS/MS analysis demonstrated that FAAH was able to hydrolyze N-benzyloctadeca-9Z,12Z-dienamide, suggesting that N-benzyloctadeca-9Z,12Z-dienamide is also a slow substrate for FAAH. These results provide useful information about the mechanism of action of Lepidium meyenii and may help with the development of new compounds with FAAH inhibitory or modulatory activity.


