Luffa aegyptiaca
Luffa aegyptiaca
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Luffa aegyptiaca
- Cat.No. Product Name CAS Number COA
-
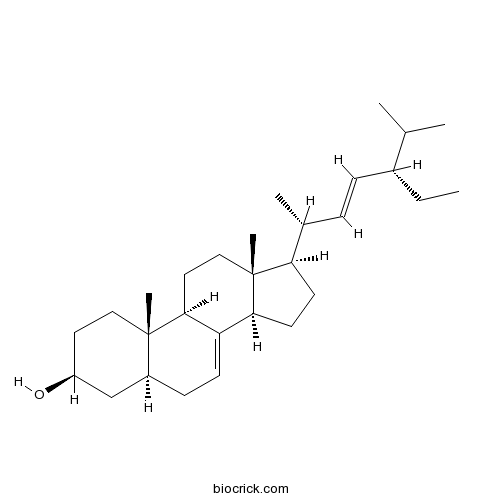
BCN8816
7,22,25-Stigmastatrienol14485-48-4
Instructions

-
BCN5564
alpha-Spinasterol481-18-5
Instructions
-
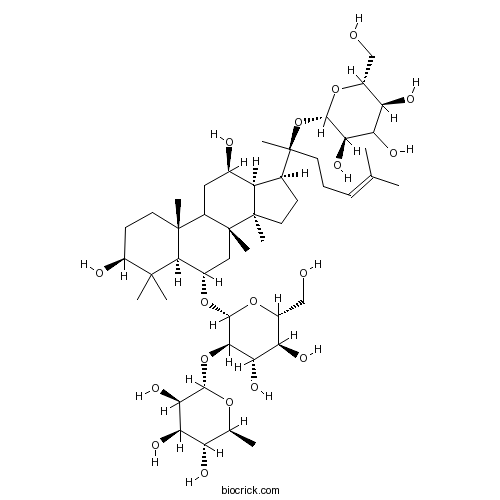
BCN1073
Ginsenoside Re52286-59-6
Instructions
-
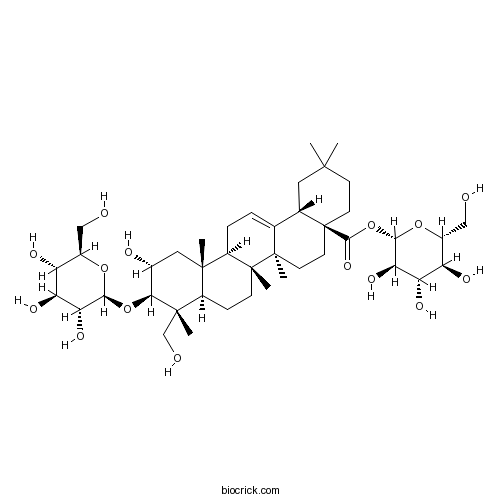
BCN7811
Lucyoside B91174-19-5
Instructions
Genotoxicity and antigenotoxicity study of aqueous and hydro-methanol extracts of Spondias mombin L., Nymphaea lotus L. and Luffa cylindrical L. using animal bioassays.[Pubmed: 27486380]
Spondias mombin (Linn), Nymphaea lotus (Linn) and Luffa cylindrica (Linn) (syn Luffa aegyptiaca Mill) are plants traditionally used as food ingredients and in the management of diseases, including cancer, in Nigeria. Despite the therapeutic potentials attributed to these plants, reports on their genotoxicity are scanty. In this study, the genotoxicity of the aqueous and hydro-methanol extract of these plants was evaluated using mouse bone marrow micronucleus and sperm morphology assays. Antigenotoxicity was assessed by the bone marrow micronucleus test. The highest attainable dose of 5 000 mg/kg according to OECD guidelines was first used to assess acute toxicity of the aqueous and hydro-methanol extracts in Swiss albino mice. For each extract, there were five groups of mice (n=4/group) treated with different concentrations of the extract as against the negative and positive control group for the genotoxicity study. In the antigenotoxicity study, five groups of mice were exposed to five different concentrations of the extracts along with 60 mg/kg of methyl methane sulfonate (MMS), which was used to induce genotoxicity. The mice were administered 0.2 mL of extract per day for 10 days in the genotoxicity and antigenotoxicity groups. Administration of each of the extracts at the concentration of 5 000 mg/kg did not induce acute toxicity in mice. At the concentrations tested, all the extracts, except aqueous S. mombin, increased micronucleated polychromatic erythrocytes. The aqueous and hydro-methanol extracts of N. lotus increased the frequency of aberrant sperm cells. All the extracts were also able to ameliorate MMS induced genotoxicity in bone marrow cells of the exposed mice. The results showed the potential of the extracts to induce somatic and germ cell mutation in male mice. The extracts also ameliorated the genotoxic effect of MMS.
Dried fruit of the Luffa sponge as a source of chitin for applications as skin substitutes.[Pubmed: 24812618]
LUFFACHITIN obtained from the residue of the sponge-like dried fruit of Luffa aegyptiaca was developed as a weavable skin substitute in this study. A chemical analysis revealed that LUFFACHITIN was composed of a copolymer containing N-acetyl-glucosamine (~40%) as a major monomer with a filamentary structure as demonstrated by both optical and scanning electron microscopy. The pulp-like white residue of the sponge-like dried fruit of Luffa aegyptiaca after treatment was then woven into a thin, porous membrane by filtration and lyophilization as a skin substitute for conducting wound-healing study on rats. The results indicated that the LUFFACHITIN membrane showed significant wound-healing enhancement (25 days to complete healing) compared to cotton gauze (>30 days), but not inferior to that of SACCHACHITIN. Furthermore, the LUFFACHITIN membrane had advantages of having a high yield, better physical properties for fabrication, and a more attractive appearance.
Regulation of pathogenicity in hop stunt viroid-related group II citrus viroids.[Pubmed: 9880036]
Nucleotide sequences were determined for two hop stunt viroid-related Group II citrus viroids characterized as either a cachexia disease non-pathogenic variant (CVd-IIa) or a pathogenic variant (CVd-IIb). Sequence identity between the two variants of 95.6% indicated a conserved genome with the principal region of nucleotide difference clustered in the variable (V) domain. Full-length viroid RT-PCR cDNA products were cloned into plasmid SP72. Viroid cDNA clones as well as derived RNA transcripts were transmissible to citron (Citrus medica L.) and Luffa aegyptiaca Mill. To determine the locus of cachexia pathogenicity as well as symptom expression in Luffa, chimeric viroid cDNA clones were constructed from segments of either the left terminal, pathogenic and conserved (T1-P-C) domains or the conserved, variable and right terminal (C-V-T2) domains of CVd-IIa or CVd-IIb in reciprocal exchanges. Symptoms induced by the various chimeric constructs on the two bioassay hosts reflected the differential response observed with CVd-IIa and -IIb. Constructs with the C-V-T2 domains region from clone-IIa induced severe symptoms on Luffa typical of CVd-IIa, but were non-symptomatic on mandarin as a bioassay host for the cachexia disease. Constructs with the same region (C-V-T2) from the clone-IIb genome induced only mild symptoms on Luffa, but produced a severe reaction on mandarin, as observed for CVd-IIb. Specific site-directed mutations were introduced into the V domain of the CVd-IIa clone to construct viroid cDNA clones with either partial or complete conversions to the CVd-IIb sequence. With the introduction of six site-specific changes into the V domain of the clone-IIa genome, cachexia pathogenicity was acquired as well as a moderation of severe symptoms on Luffa.
Differential response of human melanoma and Ehrlich ascites cells in vitro to the ribosome-inactivating protein luffin.[Pubmed: 9835461]
The cytotoxicity and inhibitory effect on proliferation of the type 1 ribosome-inactivating protein luffin purified from the seeds of Luffa aegyptiaca were investigated both in human metastatic melanoma cells and in murine Ehrlich ascites tumour cells. Results indicate that luffin from the seeds of Luffa aegyptiaca is cytotoxic to the cell lines tested, with approximately 10 times greater potency in Ehrlich cells. Luffin was found to induce an increase in cytosolic oligonucleosome-bound DNA in both melanoma and Ehrlich ascites tumour cells, the level of DNA fragmentation in the former cell line being higher than in the latter. Experiments with melanoma cells indicate that an increase in cytosolic nucleosomes could be supportive of apoptosis as the type of cell death induced by luffin.
Effect of Luffa aegyptiaca (seeds) and Carissa edulis (leaves) extracts on blood glucose level of normal and streptozotocin diabetic rats.[Pubmed: 8778506]
The present study investigates the effect of oral administration of the ethanolic extracts of Luffa aegyptiaca (seeds) and Carissa edulis (leaves) on blood glucose levels both in normal and streptozotocin (STZ) diabetic rats. Treatment with both extracts significantly reduced the blood glucose level in STZ diabetic rats during the first three hours of treatment. L. aegyptiaca extract decreased blood glucose level with a potency similar to that of the biguanide, metformin. The total glycaemic areas were 589.61 +/- 45.62 mg/dl/3 h and 660.38 +/- 64.44 mg/dl/3 h for L. aegyptiaca and metformin, respectively, vs. 816.73 +/- 43.21 mg/dl/3 h for the control (P < 0.05). On the other hand, in normal rats, both treatments produced insignificant changes in blood glucose levels compared to glibenclamide treatment.
The ribosome-inactivating, antiproliferative and teratogenic activities and immunoreactivities of a protein from seeds of Luffa aegyptiaca (Cucurbitaceae).[Pubmed: 8365647]
1. The protein isolated from Luffa aegyptiaca seeds was capable of inhibiting protein synthesis in a rabbit reticulocyte lysate system and [3H]thymidine uptake by mouse melanoma (B16) cells. 2. It also adversely affected the development of mouse embryos in culture. 3. In enzyme-linked immunosorbent assay it reacted with antisera raised against other ribosome-inactivating proteins.


