Mahonia bealei
Mahonia bealei
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
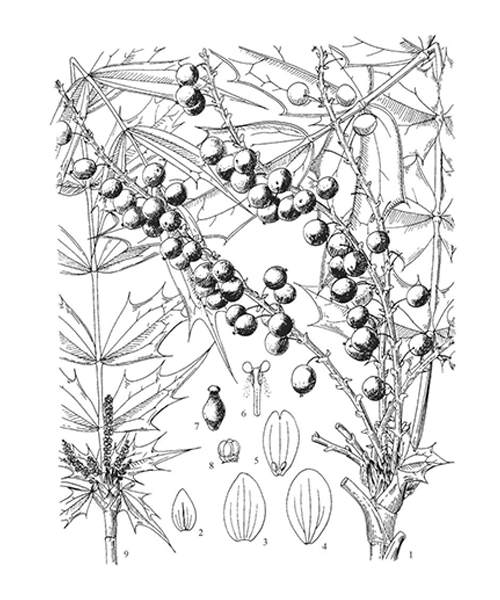
Natural products/compounds from Mahonia bealei
- Cat.No. Product Name CAS Number COA
-
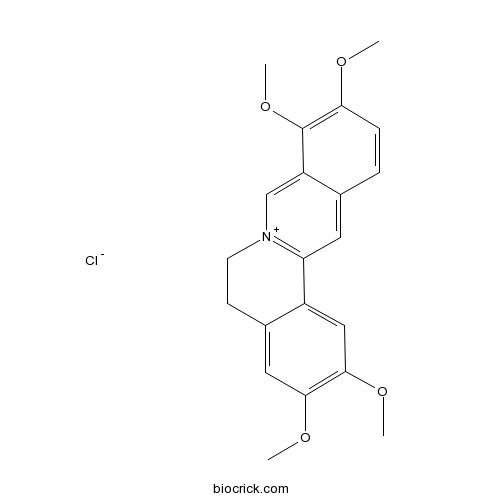
BCN5914
Palmatine hydrochloride10605-02-4
Instructions
-
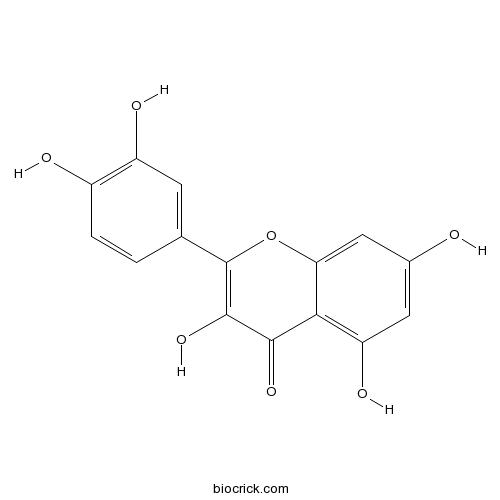
BCN6049
Quercetin117-39-5
Instructions
-
BCN5285
Palmatine3486-67-7
Instructions

-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN2851
Quercetin 3-O-beta-D-xylopyranoside549-32-6
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

Metabolites identification of berberine in rats using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry.[Pubmed: 28279930]
Berberine (BBR), the principle component for many medicinal plants such as Coptis chinensis Franch., Phellodendron chinense Schneid., and Mahonia bealei (Fort.) Carr., possesses diverse pharmacological activities, including anti-bacterial, anti-inflammatory, antitumor, hypolipidemic and antidiabetic activities. In this study, a rapid and reliable method using a five-step strategy based on the ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS), and metabolynx™ software with mass defect filter (MDF) technique was developed to investigate the metabolism of BBR. Plasma, bile, urine and feces samples were collected from rats after oral administration of BBR with a dose of 100mg/kg/day for three consecutive days and analyzed to characterize the metabolic profile of BBR. By comparing the molecular weights and MS fragmentations of the metabolites with those of the parent drug and reference standards, a total of 97 metabolites were identified, including 68 metabolites in urine, 45 metabolites in plasma, 44 metabolites in bile and 41 metabolites in feces. Demethylation, demethylenation, reduction, hydroxylation, and subsequent glucuronidation, sulfation and methylation were the major metabolic pathways of BBR in vivo.
Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer's Disease Target.[Pubmed: 27589716]
Inhibition of acetylcholinesterase (AChE) is a common treatment for early stages of the most general form of dementia, Alzheimer's Disease (AD). In this study, methanol, dichloromethane and aqueous crude extracts from 80 Traditional Chinese Medical (TCM) plants were tested for their in vitro anti-acetylcholinesterase activity based on Ellman's colorimetric assay. All three extracts of Berberis bealei (formerly Mahonia bealei), Coptis chinensis and Phellodendron chinense, which contain numerous isoquinoline alkaloids, substantially inhibited AChE. The methanol and aqueous extracts of Coptis chinensis showed IC50 values of 0.031 µg/mL and 2.5 µg/mL, therefore having an up to 100-fold stronger AChE inhibitory activity than the already known AChE inhibitor galantamine (IC50 = 4.33 µg/mL). Combinations of individual alkaloids berberine, coptisine and palmatine resulted in a synergistic enhancement of ACh inhibition. Therefore, the mode of AChE inhibition of crude extracts of Coptis chinensis, Berberis bealei and Phellodendron chinense is probably due to of this synergism of isoquinoline alkaloids. All extracts were also tested for their cytotoxicity in COS7 cells and none of the most active extracts was cytotoxic at the concentrations which inhibit AChE. Based on these results it can be stated that some TCM plants inhibit AChE via synergistic interaction of their secondary metabolites. The possibility to isolate pure lead compounds from the crude extracts or to administer these as nutraceuticals or as cheap alternative to drugs in third world countries make TCM plants a versatile source of natural inhibitors of AChE.
Palmatine from Mahonia bealei attenuates gut tumorigenesis in ApcMin/+ mice via inhibition of inflammatory cytokines.[Pubmed: 27175745]
Mahonia bealei is a Chinese folk medicine used to treat various ailments, in particular gastrointestinal inflammation‑related illnesses, and palmatine is one of its active constituents. In this study, ApcMin/+ mice, a genetically engineered model, were used to investigate the effects of palmatine on the initiation and progression of gut inflammation and tumorigenesis enhanced by a high‑fat diet. The in vitro antiproliferation and anti‑inflammation effects of palmatine were evaluated on HT‑29 and SW‑480 human colorectal cancer cell lines. The concentration‑related antiproliferative effects of palmatine on both cell lines (P<0.01) were observed. Palmatine significantly inhibited lipopolysaccharide‑induced increase in cytokine interleukin (IL)‑8 levels in the HT‑29 cells (P<0.01). In the in vivo studies with ApcMin/+ mice, after 10 or 20 mg/kg/day oral palmatine treatment, tumor numbers were significantly reduced in the small intestine and colon in a dose‑dependent manner (P<0.01 compared with the model group). The results were supported by tumor distribution data, body weight changes and organ index. The effect on survival was also dose‑dependent. Both the low‑ and high‑dose palmatine treatments significantly increased the life span of the mice (P<0.01). The gut histology from the model group showed a prominent adenomatous change along with inflammatory lesions. With palmatine treatment, however, the dysplastic changes were greatly reduced in the small intestine and colon tissue. Reverse transcription‑quantitative polymerase chain reaction analysis of interleukin (IL)‑1α, IL1‑β, IL‑8, granulocyte‑colony stimulating factor and granulocyte macrophage colony‑stimulating factor in the gut tissue showed that these inflammatory cytokines were reduced significantly following treatment (all P<0.01); serum cytokine levels were also decreased. Data suggests that palmatine has a clinical value in colorectal cancer therapeutics, and this action is likely linked to the inhibition of inflammatory cytokines.
The dichloromethane fraction from Mahonia bealei (Fort.) Carr. leaves exerts an anti-inflammatory effect both in vitro and in vivo.[Pubmed: 27167461]
Mahonia bealei has a long history of medical use in traditional Chinese medicine for the treatment of inflammatory-associated diseases. Despite numerous phytochemical and pharmacological studies, there is a lack of systematic studies to understand the cellular and molecular mechanisms of the anti-inflammatory activity of this plant.
Alkaloids from Mahonia bealei posses anti-H⁺/K⁺-ATPase and anti-gastrin effects on pyloric ligation-induced gastric ulcer in rats.[Pubmed: 25172799]
The purpose of this study was to investigate the underlying mechanism(s) of the total alkaloids (TA) from Mahonia bealei in treating pyloric ligation-induced gastric ulcers in rats. Animals were sacrificed after 19 h of the ligation. Gastric acid, peptic activities, mucin levels, H(+)/K(+)-ATPase activities and the gastrin level were analyzed. To improve the accuracy of the observations, IPP 6.0 software was introduced to measure the area of ulcer. TA (18.56 mg/kg/day, i.g.) showed an antiulcer effect by significantly decreasing the gastric ulcer areas (11.28 mm(2)) compared with model group (26.36 mm(2)). The TA ulcer inhibition ratio was 57.2%, compared with the effect of the positive control, omeprazole (62.96%). The results also showed that TA had a significant effect in inhibiting the release of H(+)/K(+)-ATPase, reducing the content of gastrin and decreasing gastric acidity on experimental animals. However, the TA had no significant effects on gastric mucus secretion and pepsin activity. Data indicated that TA had gastric ulcer protective effects by modulating the H(+)/K(+)-ATPase activity and gastrin level. TA has a potential to be developed as a pharmacological agent for the treatment of gastric ulcers.
Proteomics analysis of Mahonia bealei leaves with induction of alkaloids via combinatorial peptide ligand libraries.[Pubmed: 25109463]
Alkaloids are one of the most attractive sources for obtaining active natural products. However, alkaloids exist in the plants as the secondary metabolites with tracing amount, and there is an enormous demand for a large production. In the present study, we aimed to profile the modification of benzylisoquinoline alkaloids in Mahonia bealei seedlings under the binary stress of ultraviolet-B irradiation and dark incubation. Comparative proteomics analysis was carried out to address the underlying proteome variations that accounted for the alkaloid induction under treatment. Thirteen differential proteins were identified in the leaves under binary stress. Of note, the abundance of S-adenosyl-L-methionine synthetase was highly increased to sustain a high concentration of S-adenosyl-L-methionine for the enhanced biosynthesis of alkaloids. Additionally, we presented the application of CPLL to M. bealei leaf proteins. Three new secondary metabolism proteins and 12 additional differential proteins were identified only after CPLL treatment. Six genes in the benzylisoquinoline alkaloid biosynthesis pathway were selected to verify their variable expression using quantitative real time polymerase chain reaction. The results suggest that the benzylisoquinoline alkaloids in M. bealei leaf were increased to eliminate the adverse effect of UV-B exposure. The suppression of photosynthesis and respiratory rate may save an extra energy for the secondary metabolites, and the enhanced N-metabolism may supply sufficient primary metabolite precursors. To our best knowledge, this is the first work aimed at the secondary metabolism proteomic characterization of M. bealei using the CPLL technique. It also presented an effective and innovative process to improve the contents of alkaloids in medicinal plants for industrial production.
Absorption characteristics of the total alkaloids from Mahonia bealei in an in situ single-pass intestinal perfusion assay.[Pubmed: 25053555]
To investigate the absorption characteristics of the total alkaloids from Mahoniae Caulis (TAMC) through the administration of monterpene absorption enhancers or protein inhibitors.
The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms.[Pubmed: 23900198]
Mahonia bealei (Berberidaceae) is a frequently-used traditional Chinese medicinal plant with efficient anti-inflammatory ability. This plant is one of the sources of berberine, a new cholesterol-lowering drug with anti-diabetic activity. We have sequenced the complete nucleotide sequence of the chloroplast (cp) genome of M. bealei. The complete cp genome of M. bealei is 164,792 bp in length, and has a typical structure with large (LSC 73,052 bp) and small (SSC 18,591 bp) single-copy regions separated by a pair of inverted repeats (IRs 36,501 bp) of large size. The Mahonia cp genome contains 111 unique genes and 39 genes are duplicated in the IR regions. The gene order and content of M. bealei are almost unarranged which is consistent with the hypothesis that large IRs stabilize cp genome and reduce gene loss-and-gain probabilities during evolutionary process. A large IR expansion of over 12 kb has occurred in M. bealei, 15 genes (rps19, rpl22, rps3, rpl16, rpl14, rps8, infA, rpl36, rps11, petD, petB, psbH, psbN, psbT and psbB) have expanded to have an additional copy in the IRs. The IR expansion rearrangement occurred via a double-strand DNA break and subsequence repair, which is different from the ordinary gene conversion mechanism. Repeat analysis identified 39 direct/inverted repeats 30 bp or longer with a sequence identity ≥ 90%. Analysis also revealed 75 simple sequence repeat (SSR) loci and almost all are composed of A or T, contributing to a distinct bias in base composition. Comparison of protein-coding sequences with ESTs reveals 9 putative RNA edits and 5 of them resulted in non-synonymous modifications in rpoC1, rps2, rps19 and ycf1. Phylogenetic analysis using maximum parsimony (MP) and maximum likelihood (ML) was performed on a dataset composed of 65 protein-coding genes from 25 taxa, which yields an identical tree topology as previous plastid-based trees, and provides strong support for the sister relationship between Ranunculaceae and Berberidaceae. Molecular dating analyses suggest that Ranunculaceae and Berberidaceae diverged between 90 and 84 mya, which is congruent with the fossil records and with recent estimates of the divergence time of these two taxa.


