Morus cathayana
Morus cathayana
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Morus cathayana
- Cat.No. Product Name CAS Number COA
-
BCN8844
Mulberrofuran Q101383-35-1
Instructions
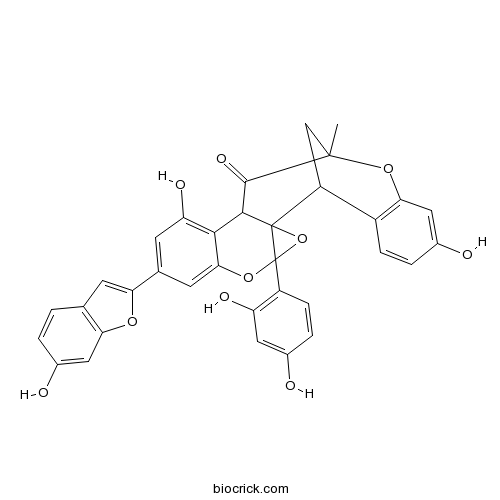
Chemical constituents of the stem bark of Morus cathayana.[Pubmed: 20552491]
Phytochemical investigation of the stem bark of Morus cathayana led to the isolation and identification of six new compounds, cathayanons F-J (1-5) and cathayanin A (6), and two known compounds, cathayanins B-C (7-8). Their structures were elucidated by spectroscopic methods. Compounds 1, 2, 3, 5, and 7 exhibited weak activities against five human cancer cell lines, with IC(50) values ranging from 4.7 to 9.8 microg/ml.
Three new Diels-Alder type adducts from the stem bark of Morus cathayana.[Pubmed: 19408152]
Three new Diels-Alder type adducts cathayanons C (1), D (2), and E (3), together with one known compound sanggenon C (4), were isolated from the stem bark of Morus cathayana. Their structures were fully elucidated by spectroscopic and chemical methods. Compound 2 showed good anti-oxidation activity with the inhibitory rate of malondialdehyde being 88% at a concentration of 10(- 6) mol/l.
2-Arylbenzofuran Derivatives from Morus cathayana.[Pubmed: 19338315]
Four new 2-arylbenzofuran derivatives, cathafurans A (1), B (2), C (3), and D (4), were isolated from the stem bark of Morus cathayana. Their structures were determined by spectroscopic methods. Compounds 2 and 3 exhibited moderate activities against five human cancer cell lines, with IC(50) values ranging from 6.17 to 9.60 microg/mL.
A new 2-arylbenzofuran from the root bark of Chinese Morus cathayana.[Pubmed: 17689036]
A new 2-arylbenzofuran, sanggenofuran B (3',5'-dihydroxy-6-methoxy-4'-prenyl-2-arylbenzofuran), from the root bark of Chinese Morus cathayana is reported.
Diels-Alder type adducts from Morus cathayana.[Pubmed: 11454350]
Two natural Diels-Alder type adducts, cathayanon A (1) and cathayanon B (2), resembling sanggenon C (4) and O (3), were isolated from the root bark of Morus cathayana. Their structures were elucidated on the basis of spectroscopic evidence. The structure of 1 was confirmed by the results of X-ray crystallographic analysis. The absolute configurations of 1 and 2 were determined as 2S, 3R,14S, 19S, 20R and 2S, 3R, 14R, 19S, 20R, respectively. The absolute configurations at C-2 and C-3 of two other known isomeric Diels-Alder adducts sanggenon O (3) and C (4) were deduced as 2R, 3S. Pharmacological tests indicated that 1 and 2 exhibited potent activities on the inhibition of HL-60 cell adhesion to BAEC at concentrations of 10(-5) mol l(-1), with inhibitory rates of 44.72 and 39.02%, respectively.
Cytotoxic flavonoids with isoprenoid groups from Morus mongolica.[Pubmed: 11429996]
A new pyranoflavanone, sanggenol L (1), a Diels-Alder type adduct regarded as a cycloaddition product of a dehydrogeranylflavanone and a prenylchalcone, sanggenol M (2), along with four new 2-arylbenzofurans with isoprenoid units, mulberrofurans W-Z (3-6), were isolated together with 10 known flavonoids from Chinese Morus mongolica. The structures of these novel compounds were elucidated by spectroscopic methods. All flavanones investigated here showed higher cytotoxicity against human oral tumor cell lines (HSC-2 and HSG) than against normal human gingival fibroblasts (HGF). Among them, the cytotoxicity of compound 2 and the Diels-Alder type flavanone sanggenon C (7) isolated from Morus cathayana were the most potent. On the other hand, seven 2-arylbenzofurans exhibited lower cytotoxicity and tumor specificity as compared with flavanones.


